Chemical looping, a low carbon technology for the fossil fuel industry, is increasingly been viewed as a competitive technology in carbon capture and storage, with the successful completion of pilot plant trials in the USA.
As the world increasingly transitions to a low-carbon economy, it is becoming important for fossil fuel-based industries to develop ways to reduce their carbon dioxide (CO2) emissions. To do this many fossil fuel-based industries, and in particular coal-based power stations, are promoting carbon capture and storage (CCS). This is where the CO2 generated from coal combustion is separated from the power station’s flue gas and sequestered for long-term storage. The advantage of CCS is that it enables existing infrastructure and industries to meet carbon emission reductions while continuing to operate into the medium-term future.
In CCS, one of the key technology barriers is developing separation technologies that can produce a pure CO2 product for sequestration. Currently, most approaches focus on separating CO2 from the flue gas being emitted from a power station’s chimney.
This is an energy intensive approach, as they are trying to separate CO2 from a range of other gases, such as nitrogen, oxygen and water vapour. Hence, up to 25% of the power station’s output can be required to separate and purify the CO2 gas. This is known as the CCS parasitic load on the power station, and the major focus of current CCS research is to reduce the parasitic load as much as possible.
A new technology
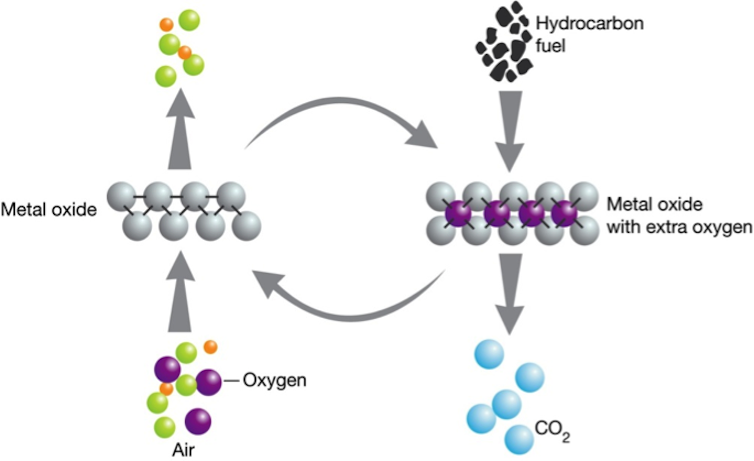
Chemical looping involves an innovative approach to deliver only oxygen to the coal combustion process, excluding other gases, such as nitrogen found in air. This enables an almost pure CO2 gas to be produced, which can then be relatively easily stored without any further major processing.
The delivery of oxygen to the combustion zone is achieved through a metal or metal oxide reaction. Small particles of metal, such as manganese or iron, are exposed to air and react with the oxygen present to form a metal oxide; this is known as oxidation. This is exactly the same process as iron rusting, however it is done at a higher temperature and inside specially designed reactors to speed up the process.
The metal oxide (or rusted metal) is then transported to the coal combustion furnace where no nitrogen is present. In chemistry the resulting reaction between the fossil fuel and metal oxide is known as a reduction reaction, where the carbon in the fuel reacts with the oxygen in the metal oxide to produce CO2 and convert the metal oxide back to the pure metal. Given that there are no other gases present, a pure CO2 flue gas is produced which is ready for sequestration storage.
Importantly, the metal particles are then recycled back to undergo oxidation in air to produce the metal oxide and the process begins again. This recycling of the metal or metal-oxide is the looping part of the technology.
This provides a major advantage in generating a technology with a low energy demand and a low parasitic load on the power station. Chemical looping has a significant advantage compared to traditional carbon capture technologies, in particular amine solvent absorption, the current technology of choice in the chemical engineering industry.
Problems to address
The successful pilot plant trials undertaken in Ohio State University clearly demonstrate the potential for the technology. However, one of the major drawbacks of the technology is the metal particles themselves.
The metal particles are like sand and they are essentially blown between the combustion furnace and oxidation reactor by the CO2 gas produced. All of the particles together are very abrasive and the plant equipment essentially experiences constant sand blasting internally as the metal or metal-oxides complete their loop.
This is a significant engineering problem, as the chemical looping plant must be made from expensive metals that can withstand the constant abrasion of the metal particles. At this stage, this makes any chemical looping plant much more expensive to construct than other carbon capture technologies, especially when compared to well-established amine solvent technology.
Chemical looping is unlikely to be one of the first generation CCS technologies that are expected to be implemented over the next decade. Instead, researchers will take that time to address the engineering problems and run larger trials. It is likely that chemical looping will a be second and third generation CCS technology, available in the coming decades.

