It can take 12 years and more than £1 billion to create a new medicine. But when we swallow a capsule, squeeze those eye drops or administer an injection, we rarely stop to think about the work that has gone into making the product. We don’t spare a moment to consider whether we are even using the medicine to its full potential.
These concerns matter, and only a more informed and better educated public will be able to address them. To help with this, a guide published by the Royal Pharmaceutical Society, New Medicines, Better Medicines, Better Use of Medicines, lays out challenges and opportunities in this sector.

Better adherence
The NHS spends around an eighth of its budget on medicines, with the number of prescriptions in primary care increasing year-on-year. However, around half of people with long-term conditions do not take their medicines as intended which leads to disease progression. This can, for instance, contribute to the development of antibiotic-resistant infections. Pharmaceutical scientists work hard to develop new and better medicines, but, if we don’t take them as intended, this hard work will be wasted.
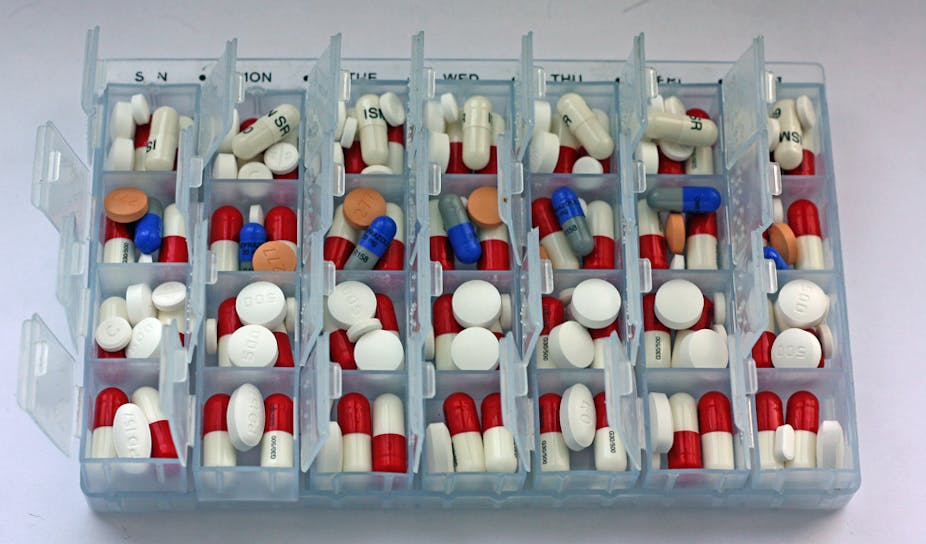
Tackling adherence is not simple. Until recently multi-compartment aids, which separate doses across a week (pictured top), were seen as an easy solution for many patients but recent guidance tells us such compliance aids are not enough. Improving people’s adherence to their medication requires a varied approach with regular interventions that are tailored to each patient.
However, even with intensive programmes in place to help people use their medicines better they still might not receive full benefit from their medicines.
Better understanding of risks
When people take medicines they often worry about side-effects. This worry is not helped when they read the patient information leaflet and are faced with a long list of possible side-effects. Research implicates adverse drug reactions, or ADRs, for causing up to 6% of hospital admissions. The majority are avoidable, but unknown because only a small proportion of ADRs are reported.
Risk of ADRs obviously increases with the number of medicines taken. About one in ten people aged over 65 take five medicines or more, making it increasingly important for clinicians to review people’s medicines. This can help identify unwanted effects and allow complex medication regimens to be simplified. With clinical trials unable to unearth every single side effect that may be experienced, it is important for patients, as well as health professionals, to send reports to the relevant medicines regulators, such as UK’s Medicines and Healthcare Products Regulatory Agency.
Better medicine
By 2050 the number of people aged over 65 in the UK is predicted to double, which could result in many people not receiving the most effective therapies available. The vast majority of clinical trials are not undertaken in children or older people, leaving us lacking evidence when using medicines in these populations.
Alongside this, the obesity crisis won’t just increase the prevalence of metabolic and cardiac diseases but may also have adverse effects on how we manage certain conditions. It is becoming apparent that the pharmacokinetics of a medicine – how it moves through the body – can differ between someone who is overweight and someone who is not. Few medicines have been sufficiently studied in those who are overweight, leaving us with an increasingly problematic knowledge gap.
Enhancing our understanding of how drugs behave in certain patient populations could help keep people healthy and out of hospital, and will be vital in ensuring personalised therapies can be used effectively.
Better informed public
And when such information is collected it should be available to the public to scrutinise, rather than being withheld as is often the case now. The AllTrials campaign is working hard to ensure clinical trial transparency, which should give us a better understanding of the risks and benefits of medicines earlier.
Patients and the public also need to be informed about the effectiveness of medicines that can be bought without a prescription. Many of these products, such as cough medicines, have not been through clinical trials so there is often little evidence of effectiveness.
Improving the use of medicines can ensure that many of these remain effective for longer. Not only would this cut the costs of treatments we will use in the future, but it will also mean that less people suffer from side-effects. Better use of medicine is a win-win for all, so what is stopping you from acting?