Standing on a walkway at Yellowstone National Park, I admired the hues of orange, blue and yellow in the sand of the Grand Prismatic Spring. A small sign nearby read “bacterial mats.” Visitors to Yellowstone may have noticed similar signs all over the park, but they’re often overlooked on the way to waterfalls, geysers, hot springs and more.
But these colorful structures at my feet were the reason I had come. Well, I needed a vacation – and what better place then Yellowstone? – but professional curiosity had a lot to do with the destination. I’m a microbiologist, and I had come to see the bacterial mats.
More commonly known as biofilms, these communities of tightly packed bacteria grow in close association with surfaces such as sand and soil. The term “biofilms” suggests a thin, two-dimensional substance, but these communities feature microscopic-scale tower-like structures crisscrossed with water channels, all of which is encased in a protective, self-produced slimy layer. The bacteria within communicate and demonstrate cooperative behavior reminiscent of primitive organs.
As visually stunning as I find these biofilms in nature, these bacterial communities can be detrimental to human health. Scientists like me are investigating how these bacterial biofilms form and behave so we can figure out new ways to manage and control them.
Biofilms are all around us
While made up of bacteria that are invisible to the naked eye, biofilms themselves can be much bigger, ranging from less than an inch to several hundred feet in size. Yellowstone is home to the most extensive and most colorful biofilms I’ve ever seen, but these bacterial communities are not unique to the park. Biofilms are found anywhere in nature, visible as stromatolites, pond scum and the slimy, slippery layer coating rocks and pebbles in streams.
And biofilms are not limited to the environment, either, since bacteria will stick to almost any surface in aqueous conditions and encase themselves with a slime matrix. Indeed, biofilms pose numerous problems to human-made materials such as ship hulls, cooling towers, sewage treatment plants, oil refineries, food processing and beverage plants, and household plumbing. You’ve likely seen them yourself while cleaning or doing repairs in your kitchen or bathroom, as a thick and slimy buildup in your drains and pipes. Biofilms can be a real nuisance, causing biofouling and corrosion.
The ubiquity of biofilms in our surroundings is supported by findings that the majority of bacteria, up to 90 percent, prefer living in surface-associated biofilm communities rather than as free-floating, individual bacteria (what we call planktonic bacteria).
So why do bacteria tend to form communities? For one thing, there’s strength in numbers. By banding together within their slimy protection, biofilm bacteria can remain in favorable locations or hosts, better withstand nutrient deprivation, stress, dessication and predation. At the same time, they benefit from increased cooperation and exchange of genetic material.
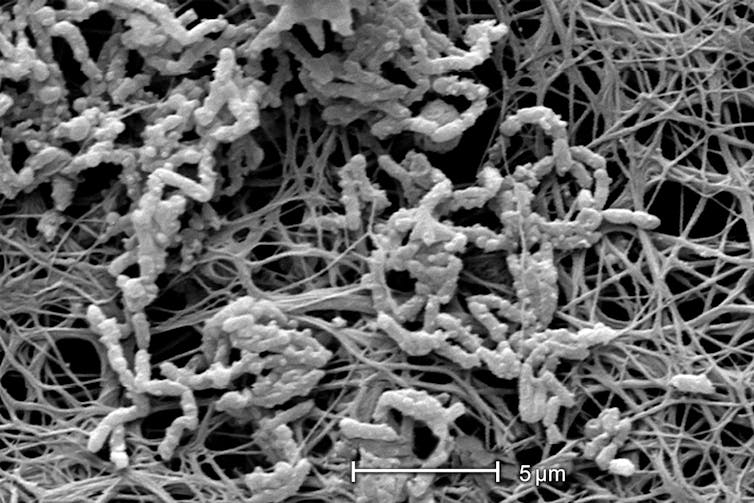
Biofilms can harm human health
Biofilms have been linked to contamination of contact lenses leading to corneal ulcers. They’re associated with dental plaque that leads to caries and periodontitis. They can infect surgical sites, the urinary tract, chronic and burn wounds and the lungs of cystic fibrosis patients. And they love to colonize medical devices such as catheters, prosthetic joints and heart valves.
According to the National Institutes of Health, more than 65 percent of chronic inflammatory and infectious diseases are due to biofilms. According to research, biofilm-related infections claim as many lives as heart attack or cancer. And they are costly, with treatment of biofilm-related infections ranging into the billions annually worldwide.
Why are we not better equipped to treat such bacterial infections? Research by my laboratory and others has demonstrated that when bacteria attach to a surface and grow as biofilms, they undergo a change, as evidenced by the genes they express and the proteins they produce. One of the consequences of this change is that biofilm bacteria become less susceptible to biocides, disinfectants and antibiotics.
Scientists think there are several reasons for this decrease in susceptibility. First, the slimy layer encasing biofilms can make it hard for disinfectants or antimicrobials to even physically reach the bacteria. Also, bacteria living in biofilms experience high stress levels while growing rather slowly, which can render most antibiotics ineffective since they only work on actively growing cells. My favorite theory is that living in a biofilm changes bacteria and their behavior; something about their mix of active genes and proteins just makes them more resilient. Whatever the contributing factors, bacteria growing in a biofilm can be up to 1,000-fold more resistant to antibiotics than the same bacteria grown planktonically.
This profound tolerance to antimicrobial agents – a hallmark of biofilms – is at the root of many persistent infections and renders biofilms extremely difficult to control in medical settings. Conventional therapies have proven inadequate in the treatment of many if not most chronic biofilm infections, mainly because they have been geared toward bacteria growing planktonically and not as biofilms.
New lines of attack against biofilms
Research suggests a promising new avenue for biofilm control: the manipulation of the biofilm lifestyle. Yes, for bacteria, being in a biofilm is a lifestyle choice.
The biofilm way of life is initiated when a few planktonic bacteria adhere to a surface. Once attached, these bacteria will divide and grow into more complex, three-dimensional structures – the biofilm. If resources become exhausted or the biofilm become too overcrowded, bacteria can escape it, as a means of survival and dissemination.
It’s the two extremes of their lifestyle, the beginning and the end, attachment and escape, that have become major foci of research endeavors looking for ways to defeat biofilms.
When it comes to controlling attachment, much research has focused on the development of new surface materials aimed at preventing the formation of biofilms on medical devices in the first place. The idea is to render devices’ surfaces nonsticky, repelling or otherwise toxic for those first pioneering bacteria. If they can’t latch on and get a toehold, no biofilm can eventually form. Surface coatings containing colloidal silver, antibiotics or micro-brushes can render medical devices inhospitable.
Likewise, the hunt is on for new chemical compounds that prevent attachment or induce escape strategies. Researchers are starting to have some success.
My own research, along with that of colleagues at Binghamton University and around the world, has led me down another path. I’ve been trying to understand how bacteria actually make these amazing biofilm structures. What proteins, polymers and factors do they need to coordinate their lifestyle? What have we learned that would let us manipulate this biofilm lifestyle?
It’s unlikely there will be only one effective treatment strategy to defeat biofilms. For one thing, many varieties of bacteria form biofilms, and they all use somewhat different strategies to enable this lifestyle. For instance, while bacteria may coordinate the formation of biofilms via chemical signals, the molecules used by bacteria such as E. coli or S. aureus to do so differ quite dramatically. Likewise with the species-specific sets of proteins required to coordinate the formation of each kind of biofilm. So as we target individual characteristics, some of our tactics work better on one group than another.
But biofilm bacteria also share some common features that we can take advantage of, including their need for communication and coordination. Building a biofilm, escaping from the biofilm or even living in a biofilm requires some sort of coordination among the millions of bacteria that make it up. They can do so by communicating with each other, using a chemical language or proteins. Jamming the bacterial language (although there are many) or interfering with their key factors required for coordination has proven to be a successful strategy to block or modify biofilm formation, at least in laboratory settings and some clinical pilot studies.
Likewise, repurposing the bacterial language has shown promise. For instance, when we co-opt the bacterial language to signal “escape from the biofilm!” we can trick biofilm bacteria into giving up their protective lifestyle and converting to planktonic cells again. The added benefit is the planktonic cells are more susceptible to antibiotics.
Controlling biofilms in the future will likely require a combination of strategies, addressing both attachment and escape, with and without the use of antibiotics and communication blockers, and likely in a manner more or less tailored toward the different bacterial lifestyles.

