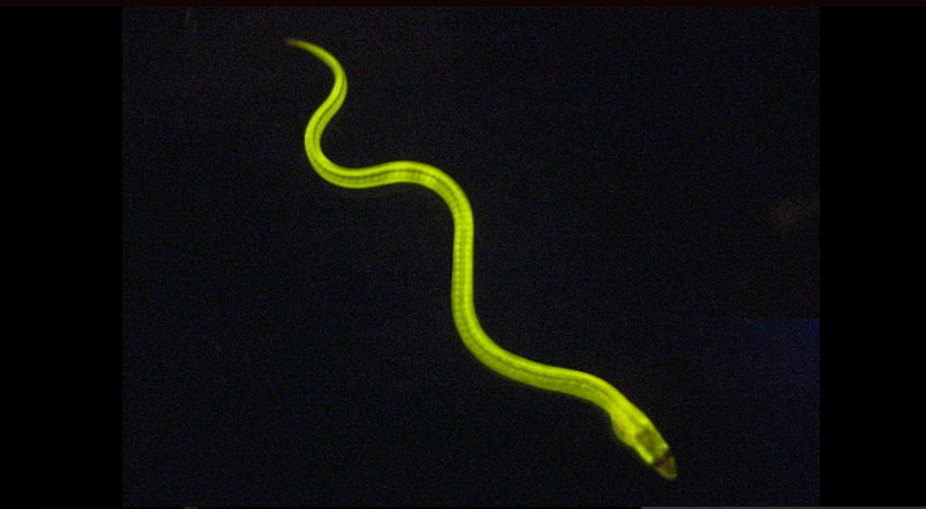
Researchers have discovered a fluorescent protein in a Japanese eel consumed as a popular sushi snack. The discovery could help develop simpler and more sensitive tests to detect jaundice and other diseases.
The idiom “seeing is believing” has been revived by biologists through the use of fluorescence microscopy, where specifically tagged proteins glow green when a laser is shone on them. This green glow allows researchers to observe phenomena at very minute scales (at some billionths of a meter).
The importance of such proteins called green fluorescent proteins (GFPs) was recognised by the 2008 Nobel Prize in Chemistry. But so far all the GFPs have been derived from non-vertebrate animals - those that lack a spinal cord - such as jellyfish and corals.
The discovery of the new fluorescent protein, named UnaG after the Japanese eel unagi, is important not just because it comes from a vertebrate animal but also because it is very different from any of the GFPs currently available.
A special glow
The first GFP was discovered in a jellyfish called Aequorea victoria almost 50 years ago. Since then tiny tweaks to this GFP and others that were discovered later on have given researchers a reliable tool to probe how a cell works.
Uncovering the cell’s molecular machinery is key to developing new medicines and tools to diagnose diseases. The use of GFPs allows scientists to pinpoint a single class of proteins among thousands that are at work in a cell.
The results of the new discovery were published in the journal Cell recently. A team led by Atsushi Miyawaki at the RIKEN Institute in Japan found UnaG when they were studying the muscle fibres of freshwater eels.
The protein was unique not just because it was found in a vertebrate animal, but also because of the way it fluoresced. Most GFPs use a chromophore, which is a part of the molecule that can absorb and emit light. Instead UnaG glows by integrating a molecule from outside the protein.
This molecule turns out to be bilirubin, which is present in eel muscles but is also formed when haemoglobin breaks down in human blood. Levels of bilirubin have been used for decades as a test to assess liver health and diagnose diseases such as jaundice. So this ability of UnaG gives it the potential to detect bilirubin and act as an indicator for liver malfunction.
Binding to bilirubin gives UnaG some more special properties that no other GFP currently has. First, it is only half the size of current GFPs, which makes it handy to tag proteins without interfering with their function. Second, most GFPs require oxygen to produce their chromophore and thus become fluorescent. UnaG does not. This means it could for the first time allow the illumination of cells in tissues where oxygen is scarce, such as some cancerous tumours.
Biology has always been driven by the ability to “see” nature. Modern tools have allowed scientists to see beyond what the naked eye could offer. And the race is on to see phenomena happening on ever smaller scales. UnaG is one big step in that direction.