Touch screens are incorporated into almost all new technologies, from smart-phones, tablet computers and personal gadgets to flat panel televisions and household appliances.
This explosion of technology is reliant on a key component: a display that is both transparent and able to conduct electrical charge.
With the ever increasing volume of devices, the future of such technologies faces substantial hurdles – material availability and raising costs. The most commonly used transparent conducting material comes from limited mineral resources, and despite almost a decade of research there is no clear replacement.
Alternatives which are getting some traction include silver nanowires, carbon nanotubes, graphene and conducting polymers.
The original transparent electrode
Traditionally, the most commonly used transparent conducting materials are doped metal oxides. Indium tin oxide (ITO) has been the market leader in this field for decades.
But the abundance of indium in the Earth’s crust is relatively low (about 0.000016% or 160ppb by weight). With the rate of mining and metal ore extraction, pure indium metal has reached a premium of US$900 a kilogram.
ITO is only transparent when coated very thinly on a device. While this is convenient in terms of saving weight and space on small gadgets, it requires high energy to deposit such a film using a technique known as physical vapour deposition.
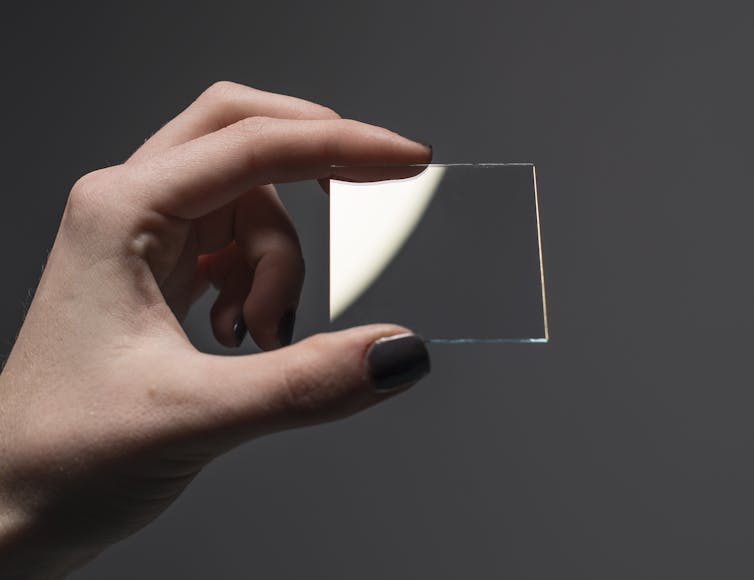
Despite its drawbacks, the desirable properties of ITO, such as optical transparency, conductivity and stability, are difficult to match.
Other metal oxide conductors such as fluorine-doped tin oxide and aluminium-doped zinc oxide can provide reasonable substitutes that almost match the properties of ITO.
While using these oxides would reduce the cost of the raw materials, there is no enhancement of the technology with new properties. Like ITO, these metal oxide films are brittle and require significant energy input to coat on substrates.
These issues have prompted researchers to look elsewhere for potential replacements which are not only much cheaper, but are more sustainable, display better performance and can be deposited on flexible substrates.
Nanowires and nanotubes
The advent of nanoparticle research provides techniques and tools to control the assembly of objects on an atomic scale.
Metal nanowires – only tens of nanometres in diameter and micrometres in length – display high conductivity. As these metal nanowires are suspended in liquid they can be sprayed or painted on almost any surface, both rigid and flexible.
By adjusting film thickness, these nanowires can match the performance of ITO. But as silver is the metal of choice in these nanowire systems there may not be any cost benefits here.
Aside from being used to make artificial muscles, carbon nanotubes are fawned over for their conducting and semiconducting properties.
These cylindrical nanostructures are made up solely of carbon atoms which means the cost of making nanotube films is quite low.
Their performance as a transparent conducting material is not always dependable at this stage, as conductivity can fluctuate wildly depending on nanotube density and the interconnections between nanotubes.
The new poster child for the nano-revolution and a close relative of carbon nanotubes, is graphene. Graphene sheets are two-dimensional structures of carbon (one layer of graphite) which can transfer charged electrons at very high speeds.
Graphene-based electrodes have the potential to be real low cost alternatives to ITO. While its use has been demonstrated in research, questions remain over the quality and reproducibility of graphene materials.
Since the production of graphene is still not very consistent, defects are commonly observed leading to less than optimal electrode performance.
Going organic
Transparent electrodes aren’t only needed to display high-resolution images, they also play a vital role in solar energy.
In photovoltaics, which convert light to electricity, a transparent conducting electrode is required to allow the passage of light through the material and to collect the current generated.
Finding a cheaper alternative is crucial to large scale solar cell technologies where competition with fossil fuel and other renewable energy resources makes cost-cutting critical to their success.
Conducting polymers may be just the material to fill this niche. With carbon atoms forming the polymer backbone, the conductivity of such materials depends on the how free the charge is to move and the conductivity of the other added materials.
As organic materials, conducting polymers can be easily processed. Researchers have even been able to print these films on a large scale at a relatively low cost.
While the desirable properties of optical transmittance and conductivity can be tuned with structural modifications, the stability of these organic materials is lower than that of ITO.
One of the most commonly used conducting polymeric material is known as poly(3,4-ethylenedioxythiophene):polystyrene sulfonate, or PEDOT:PSS for short, which has alternating units that can carry both positive and negative charges.
There is also a significant amount of research looking into hybrids or composites of the materials mentioned above.
The ultimate goal is to combine the most desirable characteristics to give a material with high conductivity and optical transparency that can be produced and processed at low cost.
Given the variety of new conducting electrodes under investigation, there is little doubt that an alternative to ITO will be found in the near future.

