The tides of Venus
Displaying 1 - 10 of 41 articles

2017 is to be the year advocacy. In January, millions took to the streets in the worldwide women’s marches. The new US president’s executive order which brought about a visa ban for citizens of a number…

You’ve all heard the Planets Suite, right? Seven classical pieces that Gustav Holst used to ‘describe’ each of the known planets. I’ve always found the Jupiter piece a bit odd – the beginning is a little…

It’s not everyday that you get to discover something new. But when you do it is a rather strange and quite brilliant feeling. You don’t really cry out ‘Eureka’ (there’s usually about a million things going…

Just think, this time last year we knew next to nothing about Pluto. It was a fuzzy blob, with even the Hubble Space Telescope struggling to make it out. Fast forward to earlier today where in a press…

Ice volcanoes have shaped my life, and until today I didn’t even know if they actually existed. Now, thanks to NASA’s New Horizons spacecraft, there’s a good chance we’ve found a frozen volcanic cone on…

This is not as odd a question as it sounds, and by next week I reckon a good lot of you will be pondering it. Why? Well the 30th September sees the opening of The Martian in Australia, director Ridley…
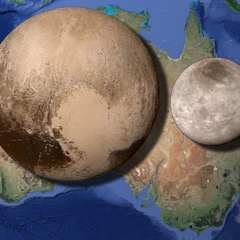
It’s now been over a month since the New Horizons spacecraft flew by one of the last unknown outposts of our solar system and although we’ve only just seen a trickle of the data it collected, it has all…

NASA has now formally started to pack its bags for the next big discovery mission, this time heading to Jupiter’s icy moon Europa. Last month NASA announced the instruments that will fly on this trip and…

As our attention is drawn to the far distant parts of our planetary neighbourhood, it is worth reminding ourselves that there’s still much to learn in our local area of the solar system. Although most…

I’ve probably lost count of the number of ‘Water On Mars’ and related headlines I’ve read over the years, but this one is worth paying attention to.
