You may not immediately think of world records when you consider chemistry, but that’s exactly what some chemists are thinking about during their research. Many, working on something called metal-organic framework (MOF) materials, are aiming to design and synthesise compounds with record-breaking surface areas.
In a recent paper in the Journal of the American Chemical Society, Northwestern University’s Omar Farha and colleagues report having synthesised compounds with a surface area of more than 7,000 metres2 /gram - measured using the Brunauer Emmett Teller (BET) technique. (The previous record was 6,210m2 /g).
To put this into context, if you could “unpack” and lay out the available surface area in a teaspoon of this material (around a gram of solid), it would cover well over an entire soccer field.
So what are MOFs?
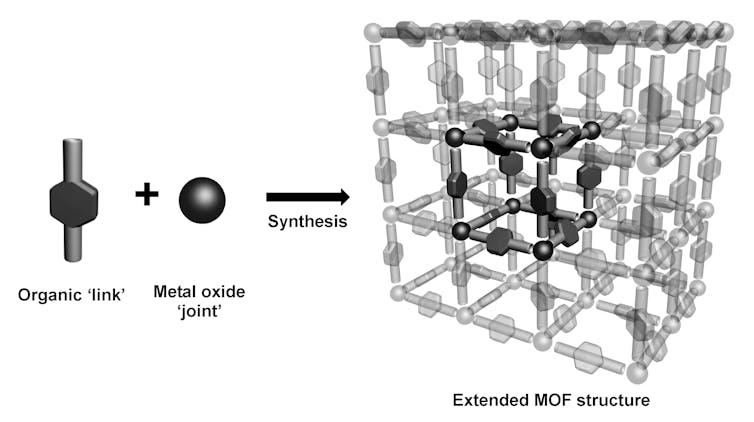
MOFs are solid materials with regular honeycomb-like structures – a little like the steel frame of a skyscraper – which can be prepared in the laboratory from organic molecules which form the links between metal atoms.
Some of the simplest links are organic compounds such as terephthalic acid – which is used to make Coke bottles – and the metal salts are often inorganic chemicals such as zinc nitrate.
The size and shape of the pores or holes in these materials can be altered by merely modifying the structure of the link or changing the metal salt.
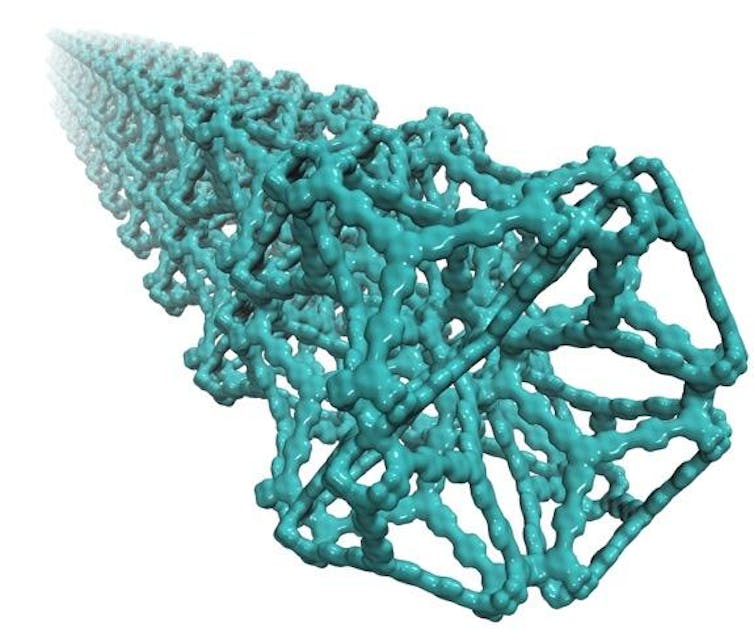
MOFs are materials that display a vast number of useful properties. Their high surface areas and open sponge-like structures make them ideal for separating mixtures of gases or for storing large volumes of a gas at pressures close to atmospheric or at ambient temperature.
And it is the allure of materials with a greater capacity for gas storage or more efficient separation potential which has driven the search for ultra-high surface area materials.
Making MOFs
There are considerable challenges in making such ultra-high surface area materials. These include:
- complications in making the required organic links
- difficulties with dissolving the starting materials to make the new MOF
- challenges associated with removing the solvent guests – molecules trapped in the MOF that support the framework – from such structures once formed.
The work of Farha and colleagues overcomes this last point by using a “supercritical CO₂ activation procedure” to remove the solvent from the MOF and make it porous. This uses CO₂ in its “fluid” state to wash the guests out prior to the CO₂ being allowed to simply evaporate from the MOF. This frees up the pore volumes and makes the surface area available for gas binding.
When the solvent that is trapped in high surface area materials such as MOFs is removed, the pull of the surface tension on the walls of the structure is considerable and can lead to collapse of the structure as it is being emptied.
Farha and colleagues used their previously developed supercritical CO₂ activation procedure to empty the ultra-high surface area material of solvent. This involves washing the material with supercritical CO₂ and then allowing the CO₂ to simply evaporate out of the pores.
The second notable breakthrough in this research is to use struts or connectors in the organic link that are more space efficient. These “acetylene” struts – which are linear atom chains – take up considerably less space that previously used “phenyl” struts. This enables the research team to make materials with even higher surface areas than was previously possible.
Farha and colleagues also note that by using poly-acetylene struts – which are extended chains of acetylene units – MOFs with even higher surface areas than those previously envisaged could be generated (up to 14,600m2 /gram).
Using MOFs
So what can we do with these ultra-high surface area materials?
When gases are absorbed into porous materials such as MOFs, the surfaces of these materials provide the highest affinity sites – that is, the gases preferentially bind to the surfaces in favour of interacting with other gas molecules.
Therefore, conceivably more gas can be adsorbed in (that is, adhered to the surface of) ultra-high surface area MOFs.
To this end, MOFs are candidate materials for on-board storage of new transport fuels such as hydrogen or methane gas. By using porous materials such as MOFs, the capacity of a tank that operates at lower pressures and more reasonable temperatures, can be enhanced.
German chemical company BASF (see video above) demonstrated this in 2006 by driving a methane-operated vehicle 28,000 miles (roughly 45,000km). The vehicle was equipped with fuel tanks that were “filled” with MOF compounds to increase their storage capacity.
High surface area MOFs might also present a solution to global warming. MOFs are currently being targeted as adsorbants for post-combustion capture of CO₂ from flue gas streams at coal-fired power stations.
Current technologies for scrubbing CO₂ are costly to run and use a considerable proportion (around 25-30%) of the energy generated by the power station. MOFs are materials that might make such technology viable.
Of course, more research and development is needed before we’ll see widespread use of MOFs in heavy-polluting industries. But the work of Farha and colleagues, and the world-record they set along the way, is a great step in the right direction.

