Some viruses are like tiny powerhouses. They are only nanometers in size, but their insides are jam-packed with DNA that is so highly pressurised that it explodes out during infection.
Herpes simplex 1 (HSV-1), the virus that causes those painful lip blisters, or cold sores, for example, has an internal pressure eight times higher than that of a car tyre. And the virus uses that pressure to literally blast its infectious DNA into human cells.
While scientists have suspected that herpes viruses did this during infection, for the first time we’ve been able to see and measure it. And the discovery of this pressure-driven infection mechanism — the first in a human virus — opens the door to new treatments for viral infections.
A controlled explosion
HSV-1 is one of a number of DNA viruses in the herpes family. As well as cold sores, members of the herpes family cause chickenpox and shingles (varicella-zoster) and genital herpes (HSV-1 and HSV-2), as well as many forms of cancer, such as Hodgkin’s lymphoma (Epstein–Barr virus or HHV-4). Some forms of herpes viruses, such as Herpes B, are lethal to humans.
Herpes simplex viruses comes in two forms: type 1 and type 2. It’s a very common infection and remains latent, although it can be activated - causing blisters or cold sores.
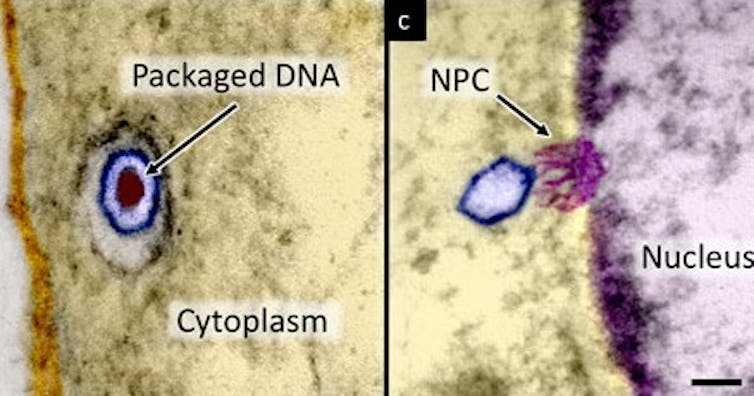
The HSV-1 virus enters the body by penetrating the outer cell membrane during infection, then travels to the cell’s nucleus where it “docks” in a small hole in the nuclear membrane and ejects its DNA load.
Our research, published in the Journal of the American Chemical Society, shows how the herpes virus does this. The virus contains DNA that is hundreds of times longer than the diameter of its tough outer shell which allows it to build up enough pressure to blast its DNA into the cell.
Many viruses pack long stretches of nucleic acid (DNA or RNA) into their protein shells. HSV-1 contains double-stranded DNA that is 400 times longer. All DNA and RNA consist of a long molecule (a polymer) which has a lot of negative charge. In viruses where the DNA is packaged tightly, the negative charges on opposite DNA strands cause strong repulsion (similar to bringing two magnets of the same polarity next to each other).
The DNA gets packaged so tightly that it bends upon itself, resulting in repulsive forces that exert tremendous energy and pressure on its outer shell. This internal pressure powers the ejection of DNA.
Along with colleagues, we discovered that we could degrade a protein in the virus called UL6. The protein acts like a plug and blocks DNA from being ejected. By degrading it we triggered the HSV-1 to eject its DNA. From this we could measure how much DNA was released and the pressure it took to do it.
It’s already known that several viruses that infect bacteria, called bacteriophages, use this high-pressure mechanism to shoot their DNA into bacteria. But despite billions of years of evolution separating human viruses and bacteriophages, our research shows that evolution has preserved this effective technique as a key step in viral infection — making it a desirable target for future treatments to defeat herpes viruses and other viruses that work the same way.
Targeting the virus in a new way
Current treatments for viral infections are highly specialised. They target proteins specific to a certain virus. But the viruses responsible for influenza, AIDS, and other infections that affect millions of people annually, for example, are quick to develop resistance to drugs that target their viral proteins. Through genetic mutations, these proteins can quickly disguise themselves and evade anti-viral drugs. This has led to a search for vulnerabilities that don’t involve viral proteins.
Also, no virus mutations can affect the negative charges in DNA. Designing drugs that interfere with viruses that use this mechanism - by reducing their DNA pressure - could limit drug resistance that develops because of rapid virus DNA mutations.

