Stem cell and cloning research using human cells remain controversial, even though it is nearly 20 years since Dolly the sheep became the first mammal to be cloned. One of the main reasons for this controversy is that such research, particularly stem cells used for therapeutic purposes, requires harvesting unfertilised eggs from human females, which brings with it a lot of ethical problems.
New research done on embryos from mice, just published in Nature, shows that there may be a way to bypass the need for unfertilised eggs. If the lead researcher Shoukhrat Mitalipov at Oregon Health and Science University is able to replicate the results in humans, it could pave the way to allowing cloning research to be done in humans. It could also boost treatments in regenerative medicine, such as creating artificial organs to replace the damaged ones.
Cloning involves creating organisms with identical copies of the genome as that of the “parent”. The usual process for doing so, called somatic cell nuclear transfer, starts with two single cells. It involves extracting the DNA-containing nucleus from a non-reproductive cell of “the parent”, a mammal, and inserting that into an unfertilised egg of that mammal whose nucleus has been removed. As the final step, the embryo so generated is placed in a female’s womb, who gives birth to this cloned child.
The unfertilised egg is key to the success of the procedure, because under the right conditions it has the capacity to start multiplying using its newly acquired DNA and create many identical copies of it – the clones. The “right conditions” involve a key part of the cell division process called metaphase. In that phase, the egg cell is getting ready to separate the two sets of chromosomes that will allow it to pass one copy of each to a daughter cell.
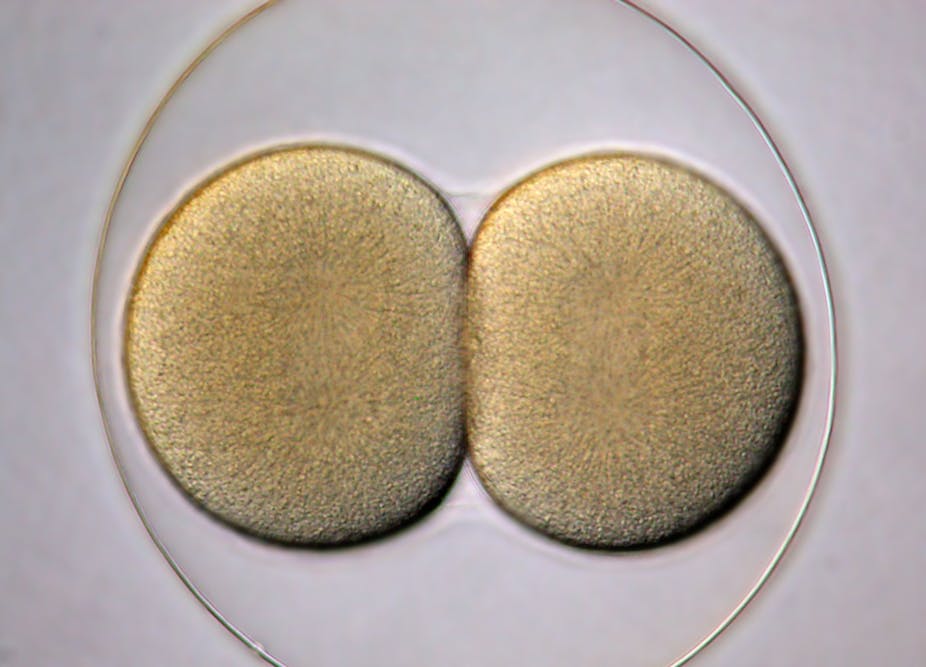
Mitalipov’s research bypasses the need for using unfertilised egg cells. He focused on a phase of cell division that comes after a fertilised egg (also called a zygote) first divides into two. He believes that this phase, called the interphase, could also have the right conditions to kickstart the cloning process.
He injected nuclei of two non-reproductive cell into both cells of the two-cell embryo that has just undergone its first cell division. He found that the conditions allowed formation of embryonic stem cells or even a clone, if that were desirable. He also found that if both the non-reproducible cell from which nucleus is removed and the two-cell embryo are synchronised to be in the interphase stage, then the success of this procedure can be dramatically increased.
Previously, scientists have used the procedure to extract stem cells from embryos, but it has stirred up a hornet’s nest among many religious groups, the bioethics community and even the United Nations. In the case of Mitalipov’s approach, no embryos need to be destroyed, which was the main concern people had raised regarding previous research. Instead, Mitalipov can use discarded or donated embryos (that were anyway not being used to give birth to a new organism), and the results can also be used to extract embryonic stem cells.
The upshot of this work is to show that the somatic cell nuclear transfer procedure isn’t as picky as we thought about the state of the cell that acts as a recipient. So, while the supply of discarded or donated embryos might be limited, the understanding that other phases in the cell cycle can be used for cellular reprogramming could go a long way to help researchers to develop newer methods to produce embryonic stem cells.
Most importantly, the main use of Mitalipov’s research is not cloning but producing stem cells of the same person. These stem cells are able to divide into many different types of mature, specialised cells, which can be used to repair different parts of the human body, and are expected to face minimum resistance from the body, since they belong to that particular individual. These stem cells are the key to regenerative medicine, the applications of which might soon be in the clinic.