Liver cancer is one of the most deadly diseases in the world with around 700,000 patients being diagnosed and more than 600,000 patients dying of the disease annually, according to the American Cancer Society. It is more common in the sub-Saharan and southeast-Asian countries, where hepatitis and alcohol abuse are the main causes. These two factors trigger liver cirrhosis, leading to cancer.
The deadly disease is now spreading to rich countries. What is particularly disturbing in there is that about 40% of the patients get liver cancer without viral infection or symptoms of alcohol abuse. But they were obese and had an intense form of fatty liver disease called non-alcoholic steatohepatitis, or NASH.
However, obesity alone cannot explain the cause of liver cancer. If it was the only risk factor, there would have been a lot more instances of liver cancer in obese patients than normal patients. But that wasn’t the case.
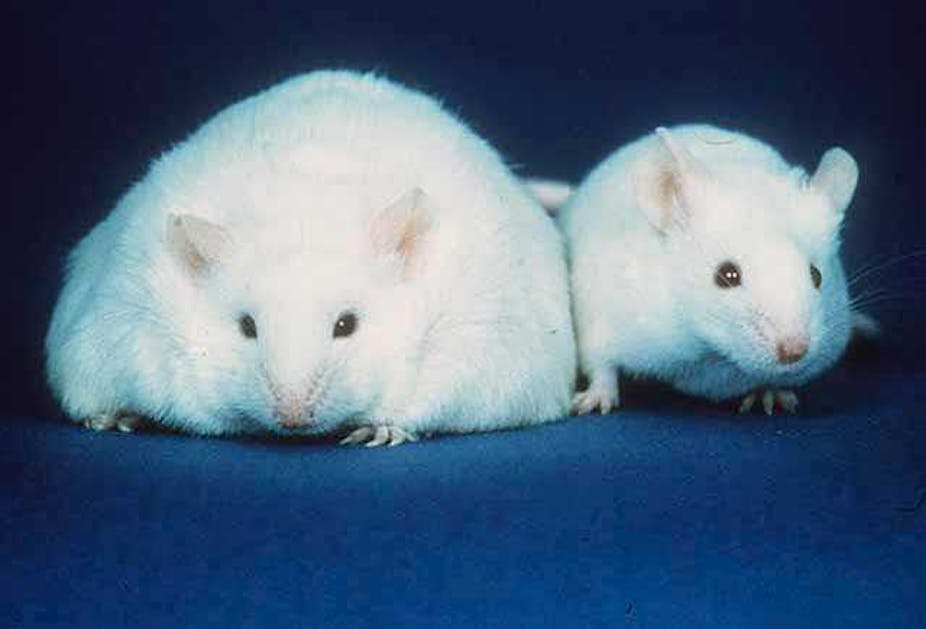
Michael Karin, professor of pharmacology, University of California at San Diego wanted to find out the exact link. In an experiment with mice, he noticed that obese mice eating a diet rich in fat did not suddenly develop liver cancer. Yet, these obese mice had a higher risk of liver cancer if they were exposed to some sort of cancer causing agent such as a chemical called diethylnitrosamine. He started looking for a naturally occurring risk factor that could tilt the balance from mere liver damage to liver cancer.
Karin suspected that a phenomenon called endoplasmic reticulum (ER) stress may have a role to play. ER stress happens whenever the cells of the liver have to work extra hard to produce more proteins. After the proteins are made, they are neatly shaped into their proper form. If there are too many proteins being made at the same time, the cell doesn’t have enough machinery available to package the proteins and it goes then into panic mode, known as ER stress.
Conditions such as hepatitis, diabetes or even long-term obesity in the patient can cause ER stress. Karin wanted to confirm whether ER stress pushed obese mice first towards intense fatty liver state, or NASH, and then ultimately towards liver disease.
Karin used a mutant strain of mice whose liver cells could artificially be made to temporarily undergo ER stress. The liver cells of these mutant mice produce an enzyme called urokinase plasminogen activator that causes ER stress. The enzyme stays within the cells for some time and then gets degraded, relieving ER stress. So the mice are completely healthy with no liver problems.
But Karin found that when the ER-stressed mice were fed a diet rich in fat, unlike the normal mice that simply turned obese, these mice started accumulating a lot of fat in their livers. Instead of temporary ER stress, the liver cells of these mice showed persistent signs of ER stress throughout their life, undergoing slow damage and death, eventually leading to liver tumours. Their results have been published in the journal Cancer Cell.
However, it is not all bad news. Karin noticed that if he could stop the process whereby ER stress causes inflammation and damage in the livers, the tumours stopped growing. He achieved this by blocking signalling molecules of the TNF pathway. The molecules of this pathway attract white blood cells called macrophages into the liver, causing inflammation.
On blocking signalling through the TNF pathway, he noticed that the liver stored less fats, making the mice look healthier. Karin thinks that drugs blocking this pathway, along with surgery and chemotherapy could be the answer to keep liver cancer under check in obese patients.