The near panic caused by the rapid spread of the Zika virus has brought new urgency to the question of how best to control mosquitoes that transmit human diseases. Aedes aegypti mosquitoes bite people across the globe, spreading three viral diseases: dengue, chikungunya and Zika. There are no proven effective vaccines or specific medications to treat patients after contracting these viruses.
Mosquito control is the only way, at present, to limit them. But that’s no easy task. Classical methods of control such as insecticides are falling out of favor – they can have adverse environmental effects as well as increase insecticide resistance in remaining mosquito populations. New mosquito control methods are needed – now.
The time is ripe, therefore, to explore a long-held dream of vector biologists, including me: to use genetics to stop or limit the spread of mosquito-borne diseases. While gene editing technologies have advanced dramatically in the last few decades, it is my belief that we’ve overlooked older, tried and true methods that could work just as well on these insects. We can accomplish the goal of producing mosquitoes incapable of transmitting human pathogens using the same kinds of selective breeding techniques people have been using for centuries on other animals and plants.

Techniques on the table
One classic strategy for reducing insect populations has been to flood populations with sterile males – usually produced using irradiation. When females in the target population mate with these males, they produce no viable offspring – hopefully crashing population numbers.
The modern twist on this method has been to generate transgenic males that carry a dominant lethal gene that essentially makes them sterile; offspring sired by these males die late in the larval stage, eliminating future generations. This method has been promulgated by the biotech company Oxitec and is currently used in Brazil.
Rather than just killing mosquitoes, a more effective and lasting strategy would be to genetically change them so they can no longer transmit a disease-causing microbe.
The powerful new CRISPR gene editing technique could be used to make transgenes (genetic material from another species) take over a wild population. This method works well in mosquitoes and is potentially a way to “drive” transgenes into populations. CRISPR could help quickly spread a gene that confers resistance to transmission of a virus – what scientists call refractoriness.
But CRISPR has been controversial, especially as applied to human beings, because the transgenes it inserts into an individual can be passed on to its offspring. No doubt using CRISPR to create and release genetically modified mosquitoes into nature would stir up controversy. The U.S. Director of National Intelligence, James Clapper, has gone so far as to dub CRISPR a potential weapon of mass destruction.
But are transgenic technologies necessary to genetically modify mosquito populations?
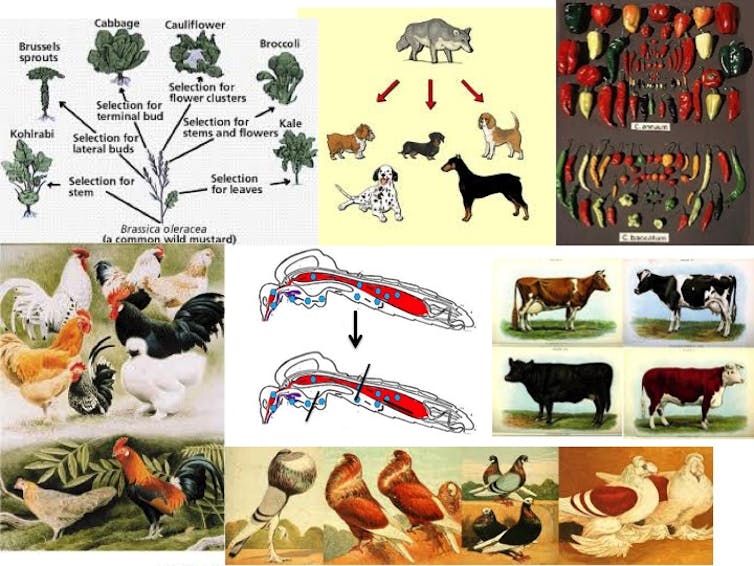
Selective breeding the old-fashioned way
Genetic modification of populations has been going on for centuries with great success. This has occurred for almost all commercially useful plants and animals that people use for food or other products, including cotton and wool. Selective breeding can produce immense changes in populations based on naturally occurring variation within the species.
Artificial selection using this natural variation has proven effective over and over again, especially in the agricultural world. By choosing parents with desirable traits (chickens with increased egg production, sheep with softer wool) for several consecutive generations, a “true breeding” strain can be produced that will always have the desired traits. These may look very different from the ancestor – think of all the breeds of dogs derived from an ancestor wolf.
To date, only limited work of this sort has been done on mosquitoes. But it does show that it’s possible to select for mosquitoes with reduced ability to transmit human pathogens. So rather than introducing transgenes from other species, why not use the genetic variation naturally present in mosquito populations?
Deriving strains of mosquitoes through artificial selection has several advantages over transgenic approaches.
- All the controversy and potential risks surrounding transgenic organisms (GMOs) are avoided. We’re only talking about increasing the prevalence in the population of the naturally occurring mosquito genes we like.
- Selected mosquitoes derived directly from the target population would likely be more competitive when released back to their corner of the wild. Because the new refractory strain that can’t transmit the virus carries only genes from the target population, it would be specifically adapted to the local environment. Laboratory manipulations to produce transgenic mosquitoes are known to lower their fitness.
- By starting with the local mosquito population, scientists could select specifically for refractoriness to the virus strain infecting people at the moment in that locality. For example, there are four different “varieties” of the dengue virus called serotypes. To control the disease, the selected mosquitoes would need to be refractory to the serotype active in that place at that time.
- It may be possible to select for strains of mosquitoes that are unable to transmit multiple viruses. Because the same Aedes aegypti mosquito species transmits dengue, chikungunya and Zika, people living in places that have this mosquito are simultaneously at risk for all three diseases. While it has not yet been demonstrated, there is no reason to think that careful, well-designed selective breeding couldn’t develop mosquitoes unable to spread all medically relevant viruses.
Fortunately, Ae. aegypti is the easiest mosquito to rear in captivity and has a generation time of about 2.5 weeks. So unlike classical plant and animal breeders dealing with organisms with generations in years, 10 generations of selection of this mosquito would take only months.

This is not to imply there may not be obstacles in using this approach. Perhaps the most important is that the genes that make it hard for these insects to transmit disease may also make individual insects weaker or less healthy than the target natural population. Eventually the lab-bred mosquitoes and their offspring could be out-competed and fade from the wild population. We might need to continuously release refractory mosquitoes – that is, the ones that aren’t good at transmitting the disease in question – to overcome selection against the desirable refractory genes.
And mosquito-borne pathogens themselves evolve. Viruses may mutate to evade any genetically modified mosquito’s block. Any plan to genetically modify mosquito populations needs to have contingency plans in place for when viruses or other pathogens evolve. New strains of mosquitoes can be quickly selected to combat the new version of the virus – no costly transgenic techniques necessary.
Today, plant and animal breeders are increasingly using new gene manipulation techniques to further improve economically important species. But this is only after traditional artificial selection has been taken about as far as it can to improve breeds. Many mosquito biologists are proposing to go directly to the newest fancy transgenic methodologies that have never been shown to actually work in natural populations of mosquitoes. They are skipping over a proven, cheaper and less controversial approach that should at least be given a shot.