Iron and steel are components of an almost limitless number of infrastructure and consumer goods. From forks to tanks, syringes to satellites, cars to computers, from buildings to the power stations that electrify them, around 1.4 billion tonnes of steel is made each year.
But manufacturing iron and steel is an energy intensive process that demands huge amounts of power and heat to reach the high temperatures required for smelting and shaping the metal. This means burning considerable amounts of fossil fuels, which in turn generates huge amounts of carbon dioxide and other greenhouse gases. It is a dirty business, which makes the need for a means to clean it up all the more pressing.
Current methods used to remove CO2 from exhaust gases require expensive “scrubbers” using caustic chemical solvents such as monoethanolamine (MEA). Instead, a new method developed for the steel industry uses the iron ore itself.
A dirty business
Producing steel from iron ore involves two stages. The first burns limestone and coke (burnt, entirely carbonised coal) as a reducing agent with iron ore in a blast furnace to form iron metal, which requires temperatures of more than 1,300°C. Natural gas can also be used to reduce iron ore. The second stage combines purifying the iron metal to form crude steel, adding small amounts of other alloying elements such as nickel, manganese or chromium to encourage certain properties. The crude steel is then shaped into a finished product such as rolled, tube or sheet steel.
About 70% of the overall carbon cost is consumed just in making the iron alone. Worldwide, each ton of steel on average emits 2.2 tonnes of CO2. After electricity-generating power plants, iron making produces the highest quantity of greenhouse gases, with cement a close third.
Considerable efforts have been made in the iron and steel industry to improve techniques in order to reduce emissions. Some require modifications to the blast furnace method, and others do not. The Ultra-Low Carbon dioxide Steel-making (ULCOS) consortium of 48 companies and organisations from 15 European countries assessed 80 different technologies. Two of these, Top Gas Recycling in Sweden, and the Hisarna process, in the Netherlands, have been tested on a pilot scale. Two others, COURSE-50 in Japan and POSCO in Korea, are also testing carbon capture and storage (CCS) programmes. But none of these technologies have been implemented on a large scale.
Using iron ore to clean steel
Our proposed carbon dioxide capture technique is based on the widely accepted carbonation-calcination cycle, which scrubs CO2 from exhaust gases by reacting it with calcium (in the form of lime) to form calcium carbonates. Instead of lime, whose ability to absorb CO2 degrades over time, our technique uses magnetite (Fe3O4) or hematite (Fe2O3) iron ores mixed with iron as an input to the carbonation process. It is recommended but pure iron is not necessarily required. Any material which is easily available and has significant iron content can also be equally good.
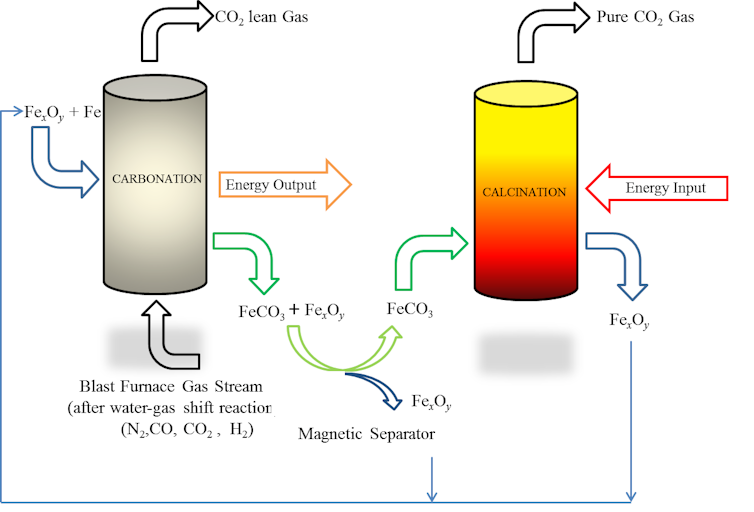
Exhaust gas from a blast furnace contains a mix of gases including carbon dioxide (CO2, 17-25%), carbon monoxide (CO, 20-28%), hydrogen, (H2, 1-5%), and nitrogen (N2, 50-55%). During carbonation, the iron oxides combine with CO2 to form siderite (FeCO3). The iron oxides can be magnetically separated from the product mixture and sent back as inputs to the carbonation process. The siderite is sent for calcination, where it is broken down into more iron oxides and pure CO2 gas. The pure CO2 gas stream can be compressed, liquefied, and put to industrial or commercial uses, or sequestered as part of a CCS strategy.
All the regenerated iron oxides can be delivered back to the carbonation reactor for the next carbonation-calcination cycle. After a large number of such cycles, the CO2 absorption capacity of iron oxide degrades. But of course as this is a steel plant, the iron oxide can still serve its original purpose of making iron and steel by being dispatched to the blast furnace. In this way, there is little or no loss of raw materials, and no chemicals and solvents required. Additionally, the calcination reaction creates heat, and the carbonation reaction requires it – the system is well balanced and efficient.
Proved in the laboratory, this technique undoubtedly has the potential to alleviate the huge costs of extra energy and raw materials needed in conventional methods, as well as reducing transportation and handling costs. Everything needed is already at the site.

