Scientists know a fair amount about the evolution of feathers in dinosaurs and birds. But less is known about the origin of hair in mammals’ ancestors. A new study by a group of researchers from South Africa’s University of the Witwatersrand used scanning technology and 3D modelling to fill in some of the gaps around mammal hair evolution. To do this, they focused on prehistoric mammal-like animals called therapsids. Natasha Joseph, science and technology editor of The Conversation Africa, chatted to lead researcher Dr Julien Benoit about their findings.
Tell us a little bit about therapsids. Why is this family of mammal-like reptiles particularly interesting? How does understanding more about therapsids influence our understanding of mammals today, for instance?
They’re considered the stem group of extant mammals – they gave birth to what we call mammals today. The Therapsida encompass a wide variety of forms, from gigantic herbivores to dreadful sabre-toothed carnivores and small insectivores. Mammals originated among the last forms, from a more inclusive group called Cynodonts. The Prozostrodontia and Probainognathia, which our research focused on, belong to Cynodonts.
Because they are our direct ancestors, a better knowledge of therapsids’ evolution helps scientists to understand our deep evolutionary roots – stretching back to more than 270 million years ago. It also helps to understand the origin of mammals’ genetic and evolutionary heritage, which could potentially unlock new horizons of research in human genetic and health sciences.
Scientists haven’t done much work until now in understanding the origin of hair in mammals’ ancestors. Why is this?
Scientists haven’t been able to do much work on the origin of hair because nobody has yet discovered a favourable deposit that preserves therapsid skin impressions.
Feathers’ fossil record starts before the origin of birds 150 million years ago. The therapsid lineage is about 100 million years older than that, so fewer of their skin remains are preserved. Skin impressions are generally rare in the fossil record. The oldest skin impression preserved with hair is 160 million years old and belongs to a mammal.
Then you have skin impressions of therapsids from the late Permian period, about 265 million years ago. Those impressions lack hair, but fossilised dung from the same period show structures that might be hairs. This is the complete and rather contradictory fossil record of hair!
You and your colleagues wanted to find out more about the origin of hair in therapsids. How did you go about doing this? What technology did you use, and how did it work?
We’ve long known that therapsids had small pits of unknown function on their snouts. These lead to a canal inside the maxillary bone for the trigeminal nerve, which is responsible for facial sensitivity.
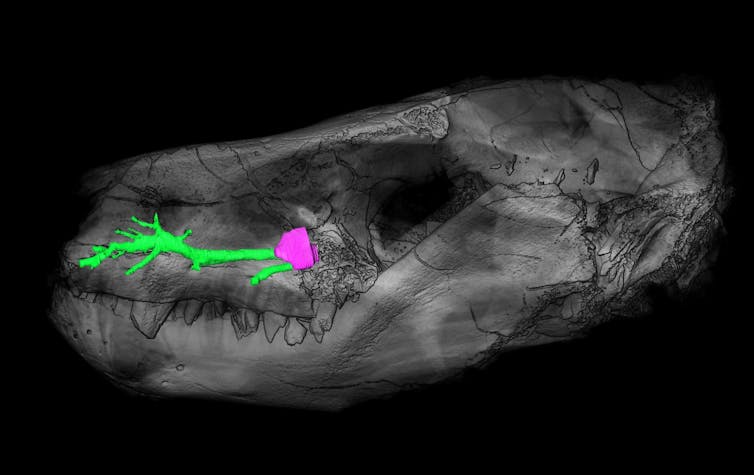
Those pits were once believed to have been for rooting whiskers. But today’s reptiles, which of course don’t have whiskers, display the same kind of pits. In the absence of direct fossil evidence for hair evolution, investigating these intriguing pits seemed to be the best way to address the evolution of hair and facial sensitivity in therapsids.
My colleagues and I used X-ray scanning technologies – CT scans – to explore these pits. CT scans allowed us to create multiple radiography of a fossil and we then reconstructed the internal structures in 3D. This technology allowed us to follow the maxillary canal in fossil therapsids and reconstruct its structure in 3D so it could be compared with other species like remaining reptiles and mammals.
For this study, we scanned 29 therapsid skulls. Most came from the collections of the Evolutionary Studies Institute at the University of the Witwatersrand in Johannesburg. This is the largest collection of South African therapsids.
You didn’t actually physically look for fossilised hair. Instead, you looked for the neural structures that innervated hairs in therapsids. What does this mean, exactly?
The first mammals were nocturnal and used whisking movements to orientate in the darkness. Mammals are able to whisk with their snouts because the trigeminal nerve is not enclosed in a long maxillary canal. It’s flexible and free to follow the snout’s movements.
In reptiles the maxillary canal completely encloses the trigeminal nerve, which is stuck in a bony canal. The snout isn’t at all flexible.
Fossils reveal that most therapsids looked like reptiles when it comes to this feature: they had inflexible snouts. Their maxillary canal disappeared as they eventually evolved toward a more mammalian condition. We were looking for the point in therapsid genealogy at which the complete maxillary canal disappeared, leaving the trigeminal nerve free to follow the whisking movements. At this point, we reasoned, whiskers would surely be present, and so we’d have a better understanding of the origin of hair in mammals.
What did you find?
Our study shows that the maxillary canal started reducing and disappeared in the earliest members of a group of therapsid named the Probainognathia. This means that probainognathians are the more likely candidates if therapsids developed whiskers before the origin of mammals. The probainognathians are also the closest relatives of modern mammals. There’s also evidence to suggest that probainognathians evolved a fur coverage.
The probainognathians have an enlarged cerebellum and a completely ossified skull roof, with no parietal foramen. This is an aperture that left the passage for the pineal gland or third eye in most therapsids. Experiments with mice and clinical observations have shown that these two traits are partly controlled by the same gene, called MSX2. This gene coincidentally controlled also for the development of mammary glands and the maintenance of fur coverage, the main two mammalian characteristics.
It would be an extraordinary coincidence if all these features – which are monitored by the same gene – appeared independently. So it’s probable that a mutation of MSX2 occurred at the evolutionary root of the Probainognathia some 245 million years ago, changed their physiology and morphology, and made them develop all the characteristics of extant mammals millions of years before the first mammal appeared.
The evolution of hair was an essential step for that of endothermy or warm-bloodedness. As I’ve said, the earliest mammals were nocturnal and couldn’t rely on their eyes to feel their environment. So, together with hearing and smell, the presence of tactile hairs on the face and body of our nocturnal ancestors played an important role in their survival and stimulated the development of the neocortex.
The development of the brain, an expensive organ that needs a lot of energy and produces a lot of heat, in turn played a role in the evolution of endothermy. Our study highlights the first step of the evolution of mammalness.

