It’s usually illegal to reuse leftover medicines in the UK for any purpose. But as a result of the coronavirus pandemic, the government has released guidance that allows this to happen in care homes and hospices, under very strict guidelines.
The guidance is to be welcomed. For the past two years, we have been calling on the government to consider changing the rules on reuse to prevent the massive amounts of waste that takes place when we throw away medicine.
The UK wasted an estimated £300 million a year of medicines in 2009, £110 million of which related to medicines returned to community pharmacies for disposal.
People are more likely to dispose of their unwanted residential medication in the household bin or down the drain than return these to pharmacies, contaminating water and soil. Prescribed medicinal waste can also harm the environment by unnecessarily inflating our carbon footprint.
A safe, sustainable system for reusing unwanted medication could make a huge difference in addressing these problems, beyond the current pandemic.
What is medicine reuse?
Medicine reuse is the idea that medication given to one patient can be taken back if it’s no longer needed and given to someone else to use instead.
Under normal circumstances, medicines dispensed in the community are not allowed to be reused, mainly because health officials worry that drugs that come back from people’s homes will have declined in quality.
Medicines, after all, are not the same as other recycled goods — to work properly, the right amount of active drug is needed, but this could potentially decrease if the medicine has been kept at the wrong temperature, or under too much light or moisture, away from the pharmacy.

The question of whether it is safe to reuse medicine is not an easy one to answer. We know from our own work on a small sample of tablets that if you take medicines out of their packaging and store them in hot and humid conditions for a month, there are at least some subtle changes.
But no one has ever fully looked at where and how medicines are generally stored when they leave the pharmacy. And we don’t actually know what (if anything) happens to medicines that are kept inside their original packaging. Because of this uncertainty, medicines returned to community pharmacies have to be disposed of instead of being reused.
Some countries including the United States and Greece do operate medication reuse schemes, mainly for benevolent reasons – the idea being that suitable medicines can re-enter the supply chain and be re-issued to others who might not otherwise afford them.
The environmental case for reuse
Medicine shortages were a growing global problem even before the pandemic began.
In 2019 alone, the UK identified supply issues for 209 medicines, including heart medicines, anti-epilepsy tablets and treatments for cancer.
Now, the pandemic has added to an existing problem, by reducing supplies from overseas manufacturers while demand for medicines has naturally increased.
The current government’s decision on the reuse of medicines, then, is a way of trying to deal with drug shortages.
Medicine reuse in care homes
What the government is suggesting now is that care homes and hospices draw up standard operating procedures in case they have to reuse some medicines.
The proposals are quite complex and need careful reading. If a medicine that is urgently required is out of stock, an alternative must be prescribed and dispensed if at all possible. If not, then the pharmacy can explore the possibility of reuse.
Importantly, the proposed medicine should no longer be needed by the original recipient, and both parties (or their representatives) must consent to the reuse. Added to this are measures to stop the possibility of cross-contamination with the coronavirus during repackaging of the reused medicine.
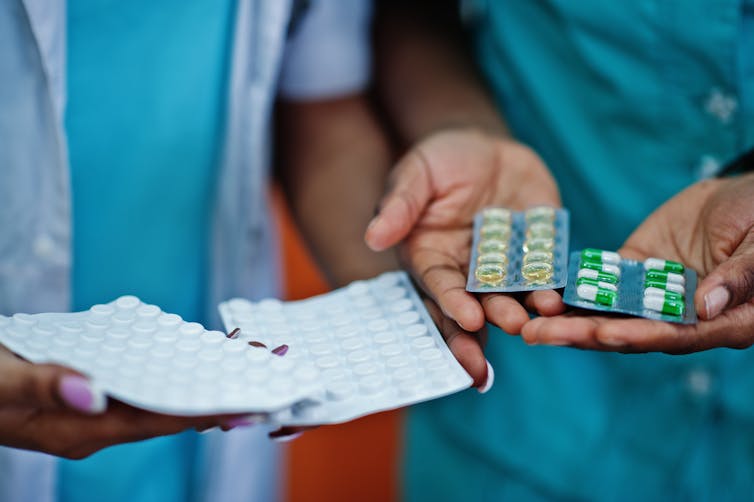
The new guidance addresses a genuine need within care homes and hospices which are already struggling on the frontlines of the pandemic, and where urgent demand for medication is likely to be for the purpose of managing someone’s pain or breathlessness at the end of their life.
Such a scenario is clearly an example where the benefit of reusing a medicine reasonably outweighs the potential risks.
A more sustainable future
It is vital that we learn from our experiences reusing medicine in care homes as we dare to look beyond the current pandemic.
At the moment, a registered health professional currently has to inspect medicines to make sure they are suitable for reuse. But whether completed in person or virtually, these checks can only provide a physical assessment, which does not guarantee the quality or amount of the medicines inside. So it will be important to find out how these inspections work in practice.
Our own ambition is to develop a technology ecosystem for the packaging of pharmaceuticals that will allow for the safe reuse of medicines in the future. This would involve creating a novel digital time temperature and humidity indicator to help with quality assurance of medicines. We have already developed a prototype using smart sensors which connect to the cloud to verify safety and enable the reuse of returned medicines.
This would provide an opportunity to explore medicine reuse not only during a pandemic but as a standard part of running a sustainable pharmacy.