Glass is a material of many faces: It is both ancient and modern, strong yet delicate, and able to adopt almost any shape or color. These properties of glass are why people use it to make everything from smartphone screens and fiber-optic cables to vials that hold vaccines.
Humankind has been using glass in some fashion for millennia, and researchers are still finding new uses for it today. It’s not uncommon to hear the oft-repeated factoid that glass is actually a liquid, not a solid. But the reality is much more interesting – glass does not fit neatly into either of those categories and is in many ways a state of matter all its own. As two materials scientists who study glass, we are constantly trying to improve our understanding of this unique material and discover new ways to use glass in the future.

What is glass?
The best way to understand glass is to understand how it is made.
The first step to make glass requires heating up a mixture of minerals – often soda ash, limestone and quartz sand – until they melt into a liquid at around 2,700 degrees Fahrenheit (1,480 Celsius). In this state, the minerals are freely flowing in the liquid and move in a disordered way. If this liquid cools down fast enough, instead of solidifying into an organized, crystalline structure like most solids, the mixture solidifies while maintaining the disordered structure. It is the atomically disordered structure that defines glass.
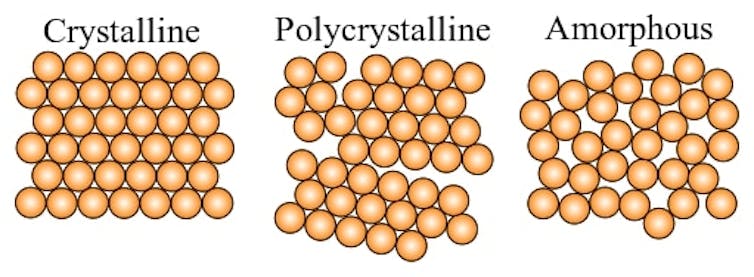
On short timescales, glass behaves much like a solid. But the liquidlike structure of glass means that over a long enough period of time, glass undergoes a process called relaxation. Relaxation is a continuous but extremely slow process where the atoms in a piece of glass will slowly rearrange themselves into a more stable structure. Over 1 billion years, a typical piece of glass will change shape by less than 1 nanometer – about 1/70,000 the diameter of human hair. Due to the slow rate of change, the myth that old windows are thicker at the bottom due to centuries of gravity pulling on the slowly flowing glass is not true.
Colloquially, the word glass often refers to a hard, brittle, transparent substance made of fused sand, soda and lime. Yet there are many types of glass that are not transparent, and glass can be made from any combination of elements as long as the liquid mixture can be cooled fast enough to avoid crystallization.
From the Stone Age to today

Humans have been using glass for more than 4,000 years, with some of the earliest uses being for decorative glass beads and arrowheads. Archaeologists have also discovered evidence of 2,000-year-old glass workshops. One such ancient workshop was uncovered near Haifa in modern Israel and dates back to around 350 C.E. There, archaeologists discovered pieces of raw glass, glass-melting furnaces, utilitarian glass vessels and debris from glass-blowing.
Modern glass manufacturing began in the early 20th century with the development of mass production techniques for glass bottles and flat glass sheets. Glass became an essential part of the electronics and telecommunications industry in the latter part of the 20th century and now forms the backbone for the internet.

Glass enabling technologies of tomorrow
Today, scientists are far beyond simply using glass as the material for a cup or a mirror. At the cutting edge of research into glass is the ability to manipulate its complex atomic structure and relaxation process to achieve certain properties.
Because glass is atomically disordered and always changing, any two points on a piece of glass are likely to have slightly different properties – whether it is strength, color, conductivity or something else. Because of these differences, two similar pieces of glass that were made in the same way using the same materials can behave very differently.
To better predict how a piece of glass behaves, our team has been researching how to quantify and manipulate the chaotic and ever-changing atomic structure of glass. Recent advances in this field have had direct benefits to existing technologies.
For example, phone screens do not crack as easily as they did in 2014 in part because new processing techniques decrease the differences in atomic bond strengths to make it harder for cracks to propagate. Similarly, internet speeds have vastly improved over the last 20 years because researchers have figured out ways to make the density of glass used for optical fibers more uniform and, therefore, more efficient at transmitting data.
A deeper understanding of how to manipulate the changing, chaotic structure of glass could lead to big advancements in technology in the coming years. Researchers are currently working on a range of projects, including glass batteries that could enable faster charging speeds and improved reliability, fiberglass wind turbines that require less maintenance than existing turbines, and improved memory storage devices.

