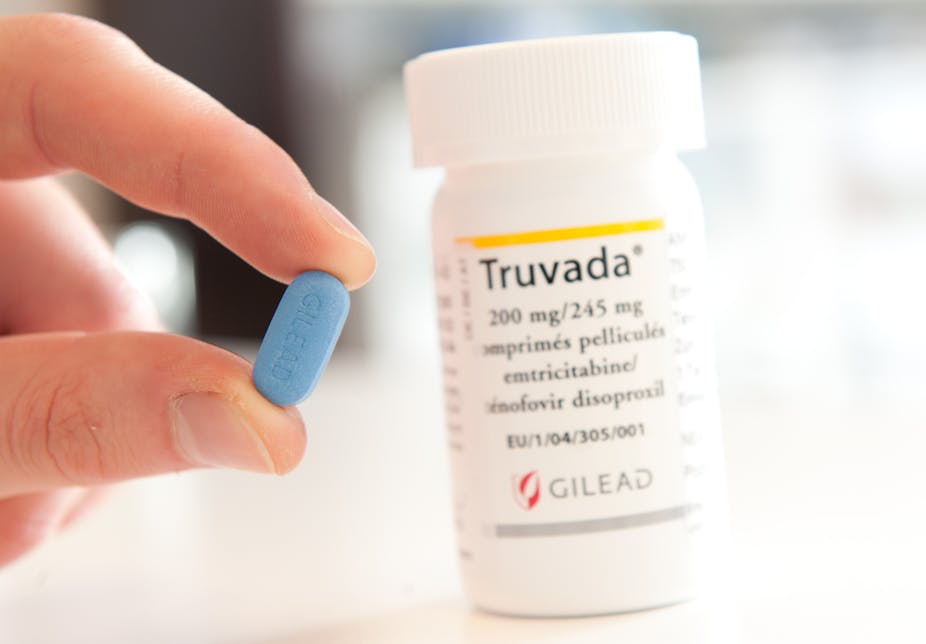
South Africa has became one of the first African countries to license a fixed-dose combination of anti-retrovirals to be used as an oral pre-exposure prophylaxis.
Pre-exposure prophylaxis, more commonly referred to as PrEP, is the use of anti-retroviral drugs by people who do not have HIV to prevent them from becoming infected. The World Health Organisation recently recommended it as an additional HIV prevention choice for people with a high risk of being infected.
The recommendations are based on studies showing that daily doses of the drug effectively reduce the HIV risk in men and women. This was irrespective of age, mode of HIV transmission or the drug regimen used. The only common factor in the level of protection was how well people adhered to the drug regimens.
The studies also showed that pre-exposure prophylaxis is safe to use in healthy populations as there was no evidence of increased side effects in trial participants.
Breaking a new frontier
South Africa’s Medicines Control Council has ruled that the drug Tenofovir disoproxil/emtricitabine (TDF/FT3), more commonly referred to as Truvada, is safe and effective for use as pre-exposure prophylaxis in the country. This paves the way for the government to issue a tender to procure the drug.
South Africa’s license is incredibly important for HIV prevention in the country as well as the region. It is a critical step to including pre-exposure prophylaxis in publicly-funded HIV prevention programmes. The licensing also means that other countries in the region are likely to follow.
Several policy, programme and procurement processes need to be followed before the drug can be distributed through the healthcare system.
The government is likely to start with pilot sites as it did with anti-retrovirals given to mothers to prevent mother to child transmission. This will help it learn:
how to identify populations who need and want to use PrEP,
what systems are needed for delivery, and
how best to monitor health so that it doesn’t overburden the health service.
A solution for young women
One of the critical steps in developing programmes is defining who will most likely benefit from them. Mathematical models suggest that it is likely to be cost-effective in settings where there are three new infections in every 100 people each year.
In Africa, the populations that have been prioritised to date are sex workers, men who have sex with men and couples where one partner is HIV infected and the other is not.
But research shows that unless HIV prevention in teenage girls and young women is prioritised, the ambitious targets set by UNAIDS to end HIV by 2030 will not happen.
Every week more than 5000 adolescent girls and young women acquire HIV. And the vast majority of them live in southern Africa. Young women are up to eight times more likely to be infected than their male peers of the same age in eastern and southern Africa. This is despite the rate of new HIV infections declining or stabilising in many other populations.
And although their HIV risk is driven in part by individual behaviour, other factors also play a role. These include:
poverty,
gender inequality and high exposure to violence,
limited economic options, and
the low social power of young people.
Adherence pitfalls
For PrEP to be effective, it will need to be integrated into existing HIV prevention services.
People at high risk for infection and who wish to start PrEP will need to be tested for HIV before they start and then every three months while taking the drugs. This will ensure that those with an early HIV infection are detected and don’t develop anti-retroviral drug resistance. They will also have their kidney function tested as Truvada can cause kidney problems in some people.
But the biggest challenge is ensuring that young people, particularly young women, who start PrEP take the pills daily as required. Those taking PrEP daily cannot miss a single dose. While two trials of pre-exposure prophylaxis in South Africa, Zimbabwe, Tanzania, Kenya and Uganda raised questions about whether young women will adhere to pre-exposure prophylaxis, recent open-label studies have shown that when populations at-risk, including young women, recognise their risk and know that pre-exposure prophylaxis is effective in preventing HIV, they are able to use it effectively.
It’s time to implement
Several pre-exposure prophylaxis demonstration projects are either underway or are being planned across southern and eastern Africa. These projects will inform the development of national policies and programmes, adding to the evidence base to understand how best to innovate, integrate and implement Truvada within a combination HIV prevention package.
The challenges of delivery will only be truly understood through implementing delivery projects. Once we have gained insights into the challenges, we will be able to refine an HIV combination prevention programme to meet the needs and preferences of teenage and young women.