In today’s electronic age, rechargeable lithium-ion batteries are ubiquitous. Compared with the lead-acid versions that have dominated the battery market for decades, lithium-ion batteries can charge faster and store more energy for the same amount of weight.
These devices make our electronic gadgets and electric cars lighter and longer-lasting – but they also have disadvantages. They contain a lot of energy, and if they catch fire, they burn until all of that stored energy is released. A sudden release of huge amounts of energy can lead to explosions that threaten lives and property.
As scientists who study energy generation, storage and conversion, and automotive engineering, we have a strong interest in the development of batteries that are energy-dense and safe. And we see encouraging signs that battery manufacturers are making progress toward solving this significant technical problem.
A new fire hazard
Urban transportation is undergoing a transformative shift toward electrification. As concerns grow in cities around the world about climate change and air quality, electric vehicles have taken center stage.
At the same time, e-bikes and electric scooters are transforming urban transit by providing convenient, low-carbon ways to navigate crowded streets and reduce traffic congestion. From 2010 through 2022, shared e-bikes and e-scooters – those owned by rental networks – accounted for more than half a billion trips in U.S. cities. Privately owned e-bikes add to that total: In 2021, more than 880,000 e-bikes were sold in the U.S., compared with 608,000 electric cars and trucks.
Battery-powered vehicles account for a small share of car fires, but controlling EV fires is difficult. Typically, an EV fire burns at roughly 5,000 degrees Fahrenheit (2,760 Celsius), while a gasoline-powered vehicle on fire burns at 1,500 F (815 C). It takes about 2,000 gallons of water to extinguish a burning gasoline-powered vehicle; putting out an EV fire can take 10 times more.
This is a major concern in large cities where electric vehicles are popular. Fire departments in New York City and San Francisco report handling more than 660 fires involving lithium-ion batteries since 2019. In New York City, these fires caused 12 deaths and more than 260 injuries from 2021 through early 2023. Clearly, there is a need for safer handling and charging practices, as well as technical improvements to batteries.

Many batteries in an EV
To understand lithium-ion battery fires, it’s important to know some basics. A battery holds chemicals that contain energy, with a separator between its positive and negative electrodes. It works by converting this energy into electricity.
The two electrodes in a battery are surrounded by an electrolyte – a substance that allows an electrical charge to flow between the two terminals. In a lithium-ion battery, for example, lithium ions carry the electric charge. When a device is connected to a battery, chemical reactions take place on the electrodes and create a flow of electrons in the external circuit that powers the device.
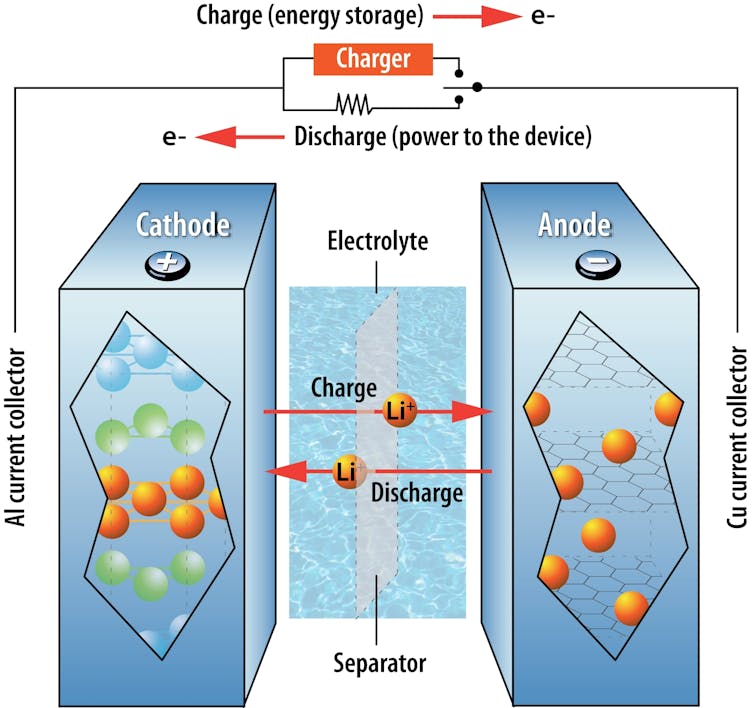
Cellphones and digital cameras can operate on a single battery, but an electric car needs much more energy and power. Depending on its design, an EV may contain dozens to thousands of single batteries, which are known as cells. Cells are clustered together in sets called modules, which in turn are assembled together in packs. A standard EV will contain one large battery pack with many cells inside it.
What causes battery fires
Typically, a battery fire starts in a single cell inside a larger battery pack. There are three main reasons for a battery to ignite: mechanical harm, such as crushing or penetration when vehicles collide; electrical harm from an external or internal short circuit; or overheating.
Battery short circuits may be caused by faulty external handling or unwanted chemical reactions within the battery cell. When lithium-ion batteries are charged too quickly, chemical reactions can produce very sharp lithium needles called dendrites on the battery’s anode – the electrode with a negative charge. Eventually, they penetrate the separator and reach the other electrode, short-circuiting the battery internally.
Such short circuits heat the battery cell to over 212 F (100 C). The battery’s temperature rises slowly at first and then all at once, spiking to its peak temperature in about one second.
Another factor that makes lithium-ion battery fires challenging to handle is oxygen generation. When the metal oxides in a battery’s cathode, or positively charged electrode, are heated, they decompose and release oxygen gas. Fires need oxygen to burn, so a battery that can create oxygen can sustain a fire.
Because of the electrolyte’s nature, a 20% increase in a lithium-ion battery’s temperature causes some unwanted chemical reactions to occur much faster, which releases excessive heat. This excess heat increases the battery temperature, which in turn speeds up the reactions. The increased battery temperature increases the reaction rate, creating a process called thermal runaway. When this happens, the temperature in a battery can rise from 212 F (100 C) to 1,800 F (1000 C) in a second.
Managing the thermal runaway problem
Methods to ensure battery safety can focus on conditions outside or inside of the battery. External protection typically involves using electronic devices, like temperature sensors and pressure valves, to ensure that the battery isn’t subjected to heat or force that could cause an accident.
However, these mechanisms make the battery larger and heavier, which can reduce the performance of the device it powers. And they may not be reliable under extreme temperatures or pressures, such as those produced in a car crash.
Internal protection strategies focus on using intrinsically safe materials for battery components. This approach offers an opportunity to address potential hazards at their source.
Making a thermal runaway in a battery pack less intense requires a mix of software and hardware improvements. Scientists are working to develop cathodes that release less oxygen when they break down; nonflammable electrolytes; solid-state electrolytes, which do not catch fire and also may help alleviate dendrite growth; and separators that can withstand high temperatures without melting.
Another solution is already in use: battery management systems. These are hardware and software packages built into battery packs that can monitor vital battery parameters, such as the state of charge, internal pressure and the temperature of the cells in the battery pack.
Just as a physician uses a patient’s symptoms to diagnose and treat their illness, battery management systems can diagnose conditions within the battery pack and make autonomous decisions to shut off batteries with hot spots, or to alter the load distribution so that any individual battery does not get too hot.
Battery chemistries are evolving rapidly, so new designs will require new battery management systems. Many battery producers are forming partnerships that bring together manufacturers with complementary battery expertise to tackle this challenge.
Users can also take steps to maximize safety. Use manufacturer-recommended charging equipment and outlets, and avoid overcharging or leaving an EV plugged in overnight. Inspect the battery regularly for signs of damage or overheating. Park the vehicle away from extremely hot or cold surroundings – for example, park in shade during heat waves – to prevent thermal stress on the battery.
Finally, in the event of a collision or accident involving an EV, follow the manufacturer’s safety protocols and disconnect the battery if possible to minimize the risk of fire or electrocution.


