The Earth formed 4.5 billion years ago, and life less than a billion years after that. Although life as we know it is dependent on four major macromolecules – DNA, RNA, proteins and lipids – only one is thought to have been present at the beginning of life: RNA.
It is no surprise that RNA likely came first. It is the only one of those major macromolecules that can both replicate itself and catalyze chemical reactions, both of which are essential for life. Like DNA, RNA is made from individual nucleotides linked into chains. Scientists initially understood that genetic information flows in one direction: DNA is transcribed into RNA, and RNA is translated into proteins. That principle is called the central dogma of molecular biology. But there are many deviations.
One major example of an exception to the central dogma is that some RNAs are never translated or coded into proteins. This fascinating diversion from the central dogma is what led me to dedicate my scientific career to understanding how it works. Indeed, research on RNA has lagged behind the other macromolecules. Although there are multiple classes of these so-called noncoding RNAs, researchers like myself have started to focus a great deal of attention on short stretches of genetic material called microRNAs and their potential to treat various diseases, including cancer.
MicroRNAs and disease
Scientists regard microRNAs as master regulators of the genome due to their ability to bind to and alter the expression of many protein-coding RNAs. Indeed, a single microRNA can regulate anywhere from 10 to 100 protein-coding RNAs. Rather than translating DNA to proteins, they instead can bind to protein-coding RNAs to silence genes.
The reason microRNAs can regulate such a diverse pool of RNAs stems from their ability to bind to target RNAs they don’t perfectly match up with. This means a single microRNA can often regulate a pool of targets that are all involved in similar processes in the cell, leading to an enhanced response.
Because a single microRNA can regulate multiple genes, many microRNAs can contribute to disease when they become dysfunctional.
In 2002, researchers first identified the role dysfunctional microRNAs play in disease through patients with a type of blood and bone marrow cancer called chronic lymphocytic leukemia. This cancer results from the loss of two microRNAs normally involved in blocking tumor cell growth. Since then, scientists have identified over 2,000 microRNAs in people, many of which are altered in various diseases.
The field has also developed a fairly solid understanding of how microRNA dysfunction contributes to disease. Changing one microRNA can change several other genes, resulting in a plethora of alterations that can collectively reshape the cell’s physiology. For example, over half of all cancers have significantly reduced activity in a microRNA called miR-34a. Because miR-34a regulates many genes involved in preventing the growth and migration of cancer cells, losing miR-34a can increase the risk of developing cancer.
Researchers are looking into using microRNAs as therapeutics for cancer, heart disease, neurodegenerative disease and others. While results in the laboratory have been promising, bringing microRNA treatments into the clinic has met multiple challenges. Many are related to inefficient delivery into target cells and poor stability, which limit their effectiveness.
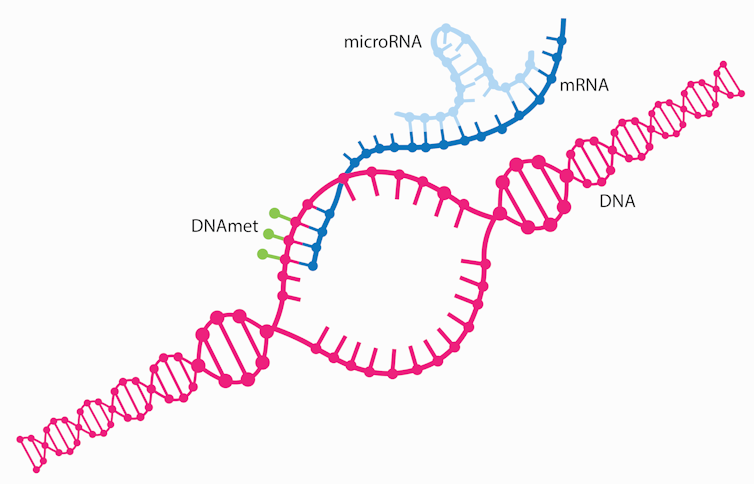
Delivering microRNA to cells
One reason why delivering microRNA treatments into cells is difficult is because microRNA treatments need to be delivered specifically to diseased cells while avoiding healthy cells. Unlike mRNA COVID-19 vaccines that are taken up by scavenging immune cells whose job is to detect foreign materials, microRNA treatments need to fool the body into thinking they aren’t foreign in order to avoid immune attack and get to their intended cells.
Scientists are studying various ways to deliver microRNA treatments to their specific target cells. One method garnering a great deal of attention relies on directly linking the microRNA to a ligand, a kind of small molecule that binds to specific proteins on the surface of cells. Compared with healthy cells, diseased cells can have a disproportionate number of some surface proteins, or receptors. So, ligands can help microRNAs home specifically to diseased cells while avoiding healthy cells. The first ligand approved by the U.S. Food and Drug Administration to deliver small RNAs like microRNAs, N-acetylgalactosamine, or GalNAc, preferentially delivers RNAs to liver cells.
Identifying ligands that can deliver small RNAs to other cells requires finding receptors expressed at high enough levels on the surface of target cells. Typically, over one million copies per cell are needed in order to achieve sufficient delivery of the drug.
One ligand that stands out is folate, also referred to as vitamin B9, a small molecule critical during periods of rapid cell growth such as fetal development. Because some tumor cells have over one million folate receptors, this ligand provides sufficient opportunity to deliver enough of a therapeutic RNA to target different types of cancer. For example, my laboratory developed a new molecule called FolamiR-34a – folate linked to miR-34a – that reduced the size of breast and lung cancer tumors in mice.
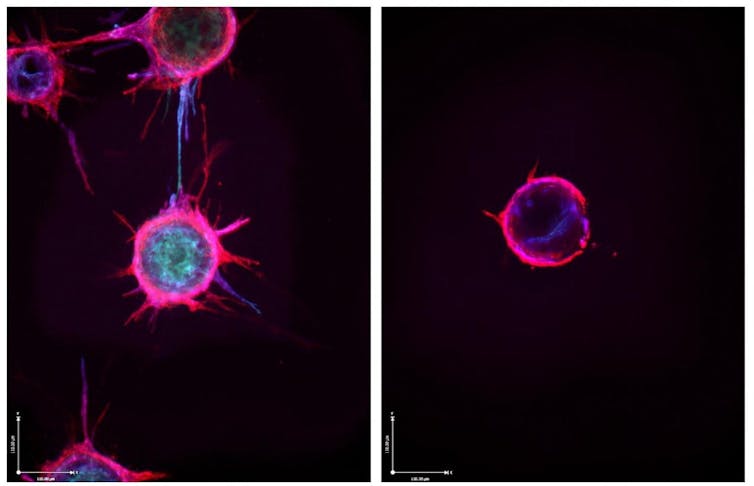
Making microRNAs more stable
One of the other challenges with using small RNAs is their poor stability, which leads to their rapid degradation. As such, RNA-based treatments are generally short-lived in the body and require frequent doses to maintain a therapeutic effect.
To overcome this challenge, researchers are modifying small RNAs in various ways. While each RNA requires a specific modification pattern, successful changes can significantly increase their stability. This reduces the need for frequent dosing, subsequently decreasing treatment burden and cost.
For example, modified GalNAc-siRNAs, another form of small RNAs, reduces dosing from every few days to once every six months in nondividing cells. My team developed folate ligands linked to modified microRNAs for cancer treatment that reduced dosing from once every other day to once a week. For diseases like cancer where cells are rapidly dividing and quickly diluting the delivered microRNA, this increase in activity is a significant advancement in the field. We anticipate this accomplishment will facilitate further development of this folate-linked microRNA as a cancer treatment in the years to come.
While there is still considerable work to be done to overcome the hurdles associated with microRNA treatments, it’s clear that RNA shows promise as a therapeutic for many diseases.