The 2013 Nobel Prize in Chemistry was yesterday jointly awarded to Martin Karplus, Michael Levitt and Arieh Warshel for developing foundation computer software that chemists today use to investigate how biological molecules work.
And while their prizewinning work was not stereotypically laboratory-based, they (among other things) revolutionised our understanding of how enzymes control the chemistry in our bodies.
The human body is one big chemical factory. The salivary glands in our mouths release digestive enzymes that help break down food into small molecules.
In the stomach and small intestine, carbohydrates, fats and proteins in our food are broken down into nutrients such as sugars, amino acids, fatty acids, vitamins and minerals, and absorbed into the bloodstream for energy, or as building blocks for new tissue.
Until the 1970s, chemical processes such as digestion were incredibly hard to follow using the tools of classical chemistry.

Enzymes are large molecules, with tens of thousands of atoms. Even when you do know how the atoms and ions are positioned in relation to each other, the structure does not tell you what these atoms and ions do.
Embedded in each enzyme is a region known as the “active site” where chemical reactions occur.
Reactions such as hydrolysis (the splitting of chemical bonds using water) occur at lightning speeds - electrons jump from one part of a molecule to another in fractions of a millisecond.
That is way too fast to follow in a test tube!
Combining quantum and molecular mechanics
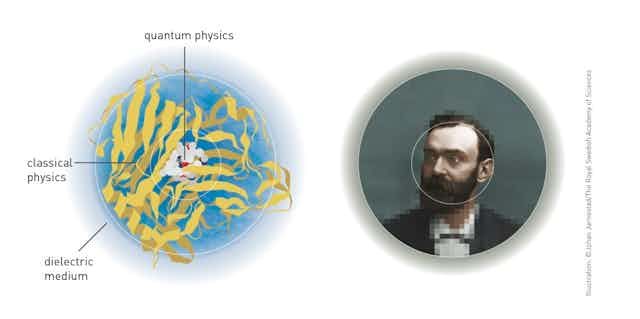
In 1974, Karplus and Warshel developed a computer model of retinal, a biological molecule that plays an essential role in eyesight. To show how retinal helps converts light into electrical signals, they found a way to combine quantum and classical physics calculations.
Karplus and Warshel’s simulation techniques changed our understanding of proteins. We now know that proteins are flexible, not rigid, molecules. Proteins interact with other molecules by changing their shape and structure as often as they need.
Warshel and Levitt then worked out how to model reactions by combining quantum and molecular mechanical calculations. In 1976, they published the first computerised model of an enzymatic reaction.
They modelled lysozyme, an enzyme that degrades bacteria cell walls, and discovered how important electrical charge effects are to lysozyme.
This was a vital development in scientists’ understanding of enzyme catalysis.
Size doesn’t matter
The discoveries made by Karplus, Levitt and Warshel mean that size is no longer an issue when simulating chemical reactions, and the strength of these modelling tools is that they are universal - they can be used to study all kinds of reactions from biochemistry to materials and industrial chemistry.
Some current researchers using such modelling include:

Debra Bernhardt, who directs the Centre for Theoretical and Computational Studies at the University of Queensland. Her team recently modelled how nanomaterials can be used to capture and store carbon dioxide.
Mark Biggs and his group at the University of Adelaide, who use molecular dynamics to study diffusion of gases in porous materials. Their results are being used to develop new materials for drug delivery.
Meredith Jordan at the University of Sydney, who is developing quantum mechanical models to study biological systems. She has investigated the chemicals that control the response of our nervous systems.
Today, Martin Karplus holds professorships at the Université de Strasbourg in France and Harvard University in the US. He is now the proud “scientific father” of children, grandchildren, and great-grandchildren all over the world having supervised more than 200 researchers who went on to lead their own research labs.
Michael Levitt is professor of cancer research at Stanford University. It has certainly been a week of Nobel glory at the Stanford School of Medicine - just two days ago the Stanford molecular neuroscientist, Thomas Südhof, won the 2013 Nobel Prize in Medicine. Levitt now aims to simulate a living organism on the molecular scale.
Arieh Warshel is a professor of chemistry at the University of Southern California in Los Angeles. Most recently, he was elected as a fellow to the American Association for the Advancement of Science.
Without their contribution, chemistry would certainly not look like it does today.