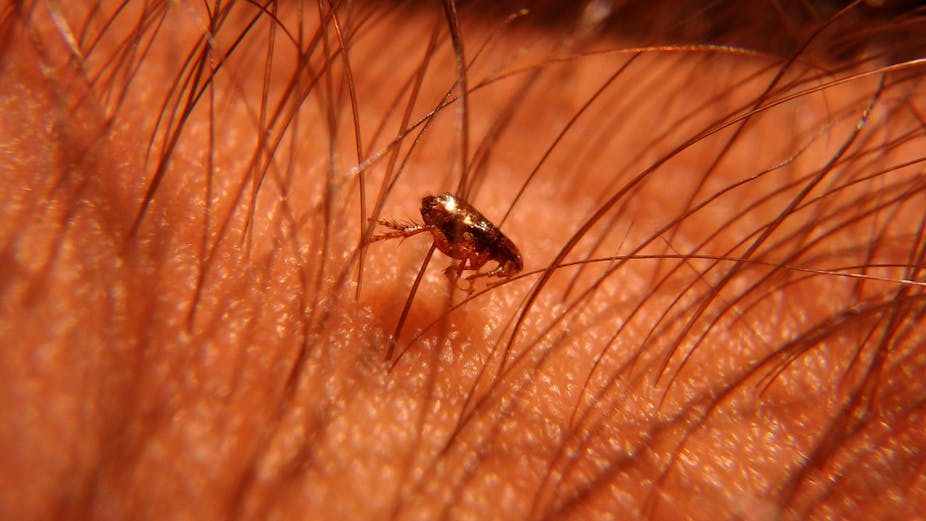
Tungiasis (sand flea disease) is a parasitic inflammatory skin condition caused by the female fleas Tunga penetrans (most prevalent) and less commonly Tunga trimamillata species. The condition is also known by names such as sand flea, jigger, chigger, nigua, kuti, bicho-do-pé, puce-chique, and pique infestation. Tungiasis occurs mainly in low- and middle-income countries and has been classified as a neglected tropical disease by the World Health Organisation (WHO). It’s one of the most widespread parasitic skin diseases in sub-Saharan Africa, Latin America and the Caribbean. Ina Skosana asked a group of public health researchers to explain.
What is tungiasis?
Tungiasis is identified by clinical examination of lesions caused by fleas burrowing into the skin, often on the toes, soles of the feet or hands. Each infected child can harbour up to hundreds of parasites – usually on their feet and hands. It typically presents as single or multiple itching red-brownish spots in the early stage. The mature stage of the disease presents as a white or yellow patch with a central black dot. And it presents as a black crust surrounded by necrotic tissue in the late stage when the flea is dead.
Tungiasis is characterised by itching, pain, and inflammation. Left untreated, the inflammation and secondary bacterial infections can have devastating effects. It can lead to deformation and loss of nails, disfigurement of the feet, death of surrounding tissue, tetanus, gangrene, sepsis and even auto-amputation in extreme cases.
Direct person-to-person transmission of tungiasis is not possible. This is because the parasite must go through off-host phases of its life cycle in the soil before infecting another human. The female parasite starts the cycle by burrowing into the host skin and feeding on host blood. This allows it to increase dramatically in size. It then expels eggs to the ground. The eggs develop in the environment, larvae hatch, develop into pupae, and finally the fleas emerge and find a host. The cycle ends with natural death of the female embedded parasite within the skin.
Who gets tungiasis?
Tungiasis affects both humans and animals. In endemic areas, children between the ages of 5 and 14 are usually most heavily affected. Prevalence rates can be as high as 97% in children, compared to 60% in the general population in highly endemic resource-poor communities in South America and in sub-Saharan Africa. It’s also one of the most common conditions of travellers returning from endemic areas.
Tungiasis has long been neglected by both the scientific community and health care providers. As a result, accurate estimates on the global burden of the disease are limited. The WHO estimates that 20 million people are at risk of developing tungiasis in South America. In 2014, an estimated 1.4 million Kenyans suffered from the condition and 10 million were at risk. There were 265 deaths due to the disease in 2011 in Kenya, according to a local non-governmental organisation. Considering many cases remain unreported, it’s highly likely that the number of deaths is much higher.
In Uganda, tungiasis affected 2.4 million people and six million others were at risk in 2012.
A 2015 Kenyan study, involving 20 primary schools, showed that about 65% of students with severe tungiasis had difficulty walking.
Another study in Kenya showed that tungiasis had a moderate to very large impact on the quality of life of the majority (78%) of affected children. This is attributed to difficulty in walking, sleep disturbance and concentration difficulties in class.
How can it be treated?
There’s no approved standard drug therapy for tungiasis. In fact, the most common current treatment is surgical removal of the embedded fleas with a sterile needle and disinfection of the residual skin. But in resource-poor communities this practice is frequently performed unhygienically. This almost inevitably leads to additional bacterial infections and more inflammation. It can also lead to the transmission of viruses like HIV, Hepatitis B and Hepatitis C.
A range of parasiticides and chemical agents have been explored for treating tungiasis. But there’s little data to support their safety and effectiveness.
Recently concluded small clinical studies in Kenya and Uganda showed considerable benefits, with limitations. A topical treatment using dimeticone (NYDA®; a synthetic silicon oil with varying viscosity) showed 78% efficacy in Kenya and 95% in Uganda. It works by suffocating the embedded parasite – otherwise it’s not lethal to the parasite, its eggs or secondary bacterial pathogens, and is unlikely to offer sustainable, long-term treatment outcomes.
Locally known repellents from natural resources (neem, coconut oils, castor oil, palm oil and Aloe Vera) have also been explored. Some countries, such as Kenya, have even included them in local government guidelines.
While researchers have been investigating new treatments, none has shown clear promise as a practically viable and clinically-proven therapeutic solution.
More importantly, it’s almost impossible to eliminate tungiasis in endemic areas without improving people’s living conditions. Nonetheless, greater focus should be given to developing new formulations or re-purposing currently available topical antimicrobial candidates with combined parasiticidal, ovicidal, antibacterial and anti-inflammatory activities, to address this truly neglected yet important clinical condition.