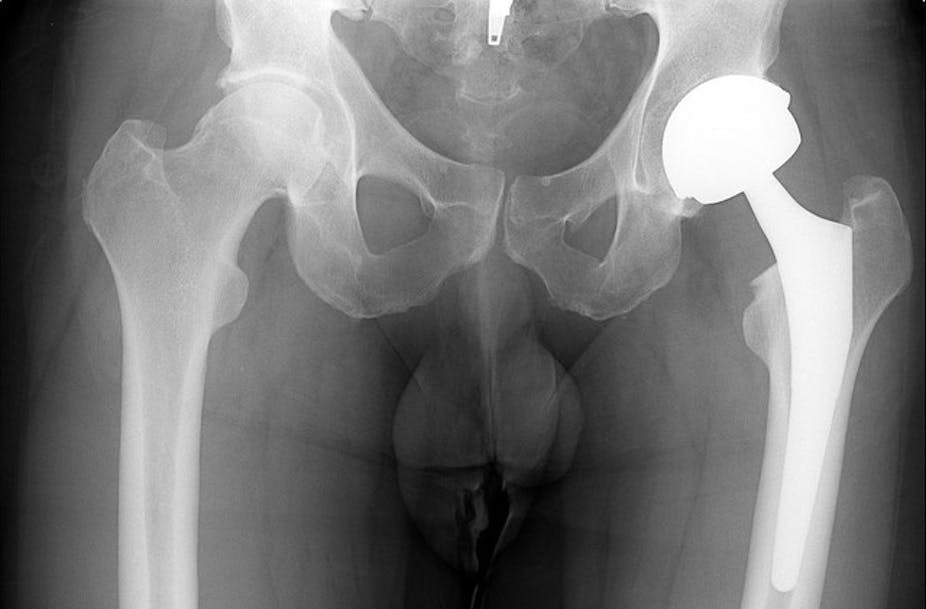
With joint replacement surgery becoming increasingly common, the flap over a large recall of De Puy hip implants has thousands of Australians worried about the quality and longevity their own hip replacements. Around 5,500 Australians received these De Puy hip implants before they were recalled last year.
Many patients became ill when the chromium and cobalt metal leached from the device into surrounding tissue. The faulty product permanently disabled some of those affected and required many others to undergo repeat surgery, costing the health-care system hundreds of thousands of dollars in avoidable costs.
The Senate’s Community Affairs References Committee, which was tasked with investigating how this failure occurred, completed its report this week and pointed the finger at the failings of Johnson & Johnson, the parent company of DePuy, and Therapeutic Goods Administration (TGA).
The Committee was “shocked” to hear about the poor health outcomes of patients who had received the implants and made 18 recommendations to ensure such a tragedy wasn’t repeated. Many of the technical recommendations boil down to a debate about the testing requirements for implantable devices before they are sold to hospitals.
Regulation and surveillance
The TGA’s current regulatory system favours minimal testing before the product is marketed and intensive post-marketing scrutiny to determine if a prosthesis causes problems.
In theory, this process is supposed to allow companies to quickly and cheaply bring products to market and allow consumers to have timely access to the best available products, with minimal red tape.
The drawback is that poorly designed or otherwise problematic implants are only identified when problems are reported to post-marketing surveillance systems such as the National Joint Replacement Registry (NJRR), or when individual surgeons report faults directly to the TGA. By this time, the product may have been implanted many thousands of times, as was the case with De Puy.
One of the key recommendations of the report is to raise the level of evidence required before the product is available to consumers. This transfers more of the responsibility for safety onto the sponsoring company and is likely to reduce the risk of faulty products being implanted.
But on the downside, it may reduce the number of devices available and delay the use of new and better devices. This is foreseeable as clinical trials are expensive and labour-intensive, and the costs will have to be passed onto consumers. Some manufacturers may even not bother marketing new and better products in Australia if they can have them listed more cheaply (and therefore more profitably) in other countries.

Safety first
In adopting a more conservative approach to allowing new products to enter the market, independent Senator Nick Xenophon, who submitted a minority report to the Committee, called for a French-style model. Under such a scheme, regulatory approval to market a product depends on the product-sponsor producing clinical evidence that their device is at least equal in performance to currently approved devices in the same class.
Such a test is already used as part of the Pharmaceutical Benefits Scheme approval process for medications.
In addition to making it harder to get devices approved, the report recommends beefing up post-marketing surveillance to detect faulty products. It advises the TGA to work more closely with the NJRR – and this relationship has already improved since the DePuy recall. As of November 2010 there were 11 additional prostheses under active monitoring.
The Committee recommended the TGA improve its communications with both health professionals and consumers about medical device issues. In this respect, it echoes the recent Transparency Review of the TGA, which highlighted how difficult it was for consumers and health providers to get information about suspect products.
The Transparency Review also slammed the internal processes of the TGA for slowing down the regulatory response to new information and following up on established problem areas. As the Committee points out, getting on with the recommendations from this review is critical to improving device regulation.
Another important Committee recommendation was to tighten scrutiny of marketing inducements offered by companies to individual surgeons or healthcare decision-makers, to bring the device regulations into line with those that apply to medicines.
A better system
As the Australian population ages, the demand for medical technology will continue to rise. The Senate Committee report clearly identifies that our current system of regulating medical devices is in need of an overhaul if more incidents like the De Puy affair are to be avoided.
Once again, there are calls for the TGA to step up an better perform its regulatory responsibilities. But money must be found from the companies that market devices, as well as from government, to provide the TGA with the regulatory powers and resources to do its job.
The Committee identifies the complexities of the situation and acknowledges such reform won’t be easy. But now is the time for the government to act on its recommendations.