Plato’s cure for headaches involved:
a certain leaf, but there was a charm to go with the remedy; and if one uttered the charm at the moment of its application, the remedy made one perfectly well; but without the charm there was no efficacy in the leaf.
We would now call Plato’s “charm” a placebo. Placebos have been around for thousands of years and are the most widely studied treatments in the history of medicine. Every time your doctor tells you that the drug you take has been proved to work, they mean that it has been proved to work better than a placebo. Every tax or insurance dollar that goes towards a treatment that is “proved” to work is proved to work because it is (supposed to be) better than a placebo.
Despite their importance, doctors are not allowed to use placebos to help patients (at least, officially), and there are debates about whether we still need them in clinical trials. Yet the science of placebos has evolved to the point where our views should – but have not – changed our prejudice against placebos in practice and the privileged position of placebo controls in clinical trials.
In this whistle-stop tour of the history of placebos, I will show what progress has been made and suggest where knowledge of placebos might go in the near future.
From pleasing prayers to pleasing treatments
The word “placebo”, as it is used in medicine, was introduced in Saint Jerome’s fourth-century translation of the Bible into Latin. Verse 9 of Psalm 114 became: placebo Domino in regione vivorum. “Placebo” means “I will please”, and the verse was then: “I will please the Lord in the land of the living.”
Historians are keen to point out that his translation isn’t quite correct. The Hebrew transliteration is iset’halekh liphnay Adonai b’artzot hakhayim, which means, “I will walk before the Lord in the land of the living.” I think historians are making much ado about not much: why would the Lord want to walk with anyone who wasn’t pleasing? Still, arguments about what placebos “really” are continue.

At that time, and even today, the mourning family provided a feast for those who attended the funeral. Because of the free feast, distant relatives, and – this is the important point – people who pretended to be relatives attended the funeral singing “placebo”, just to get the food. This deceptive practice led Chaucer to write, “Flatterers are the Devil’s chaplains, always singing Placebo.”
Chaucer also named one of the characters in The Merchant’s Tale, Placebo. The protagonist of the tale is Januarie. Januarie was a wealthy old knight who desired recreational sex with a younger woman called May. To legitimise his desire, he considers marrying her. Before making his decision, he consults his two friends Placebo and Justinius.
Placebo is keen to gain favour with the knight and approves of Januarie’s plans to marry May. Justinius is more cautious, citing Seneca and Cato, who preached virtue and caution in selecting a wife.
After listening to them both, Januarie tells Justinius that he didn’t give a damn about Seneca: he marries May. The theme of deception arises here, too, because Januarie is blind and does not catch May cheating on him.
In the 18th century, the term “placebo” moved into the medical realm when it was used to describe a doctor. In his 1763 book, Dr Pierce describes a visit to his friend, a Lady who was ill in bed. He finds “Dr. Placebo” sitting at her bedside.
Dr Placebo had impressive long curly hair, he was fashionable and he carefully prepared his medicine at the patient’s bedside. When Dr Pierce asks his friend how she was doing, she replies: “Pure and well, my old friend the Doctor has been just treating me with some of his good drops.” Pierce seems to imply that any positive effect Dr Placebo had was due to his great bedside manner, rather than the actual contents of the drops.
Eventually, the word “placebo” started being used to describe treatments. The Scottish obstetrician William Smellie (in 1752) is the first person I’m aware of who uses the term “placebo” to describe a medical treatment. He wrote: “it will be convenient to prescribe some innocent Placemus, that she may take between whiles, to beguile the time and please her imagination”. (“Placemus” is another form of the word “placebo”.)
Placebos in clinical trials
Placebos were first used in clinical trials in the 18th century to debunk so-called quack cures. Which is paradoxical because the so-called “non-quack” cures at the time included bloodletting and feeding patients the undigested material from the intestines of an oriental goat. These were considered to be so effective that no trials were needed.
The earliest example I’m aware of where a placebo control was used is in a trial of “Perkins tractors”. In the late 18th century, an American doctor called Elisha Perkins developed two metal rods he claimed conducted what he called pathogenic “electric” fluid away from the body.
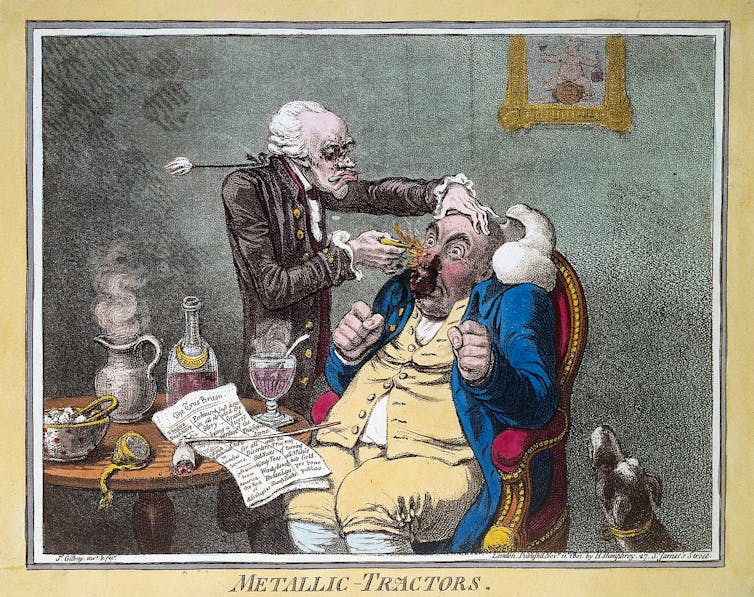
He received the first medical patent issued under the Constitution of the United States for his device in 1796. The tractors were very popular, and even George Washington is said to have bought a set.
They reached Britain in 1799 and became popular in Bath, which was already a hub for healing because of its natural mineral waters and associated spa, which have been used since Roman times. Dr John Haygarth, however, thought tractors were bunk and proposed to test their effects in a trial. To do this, Haygarth made wooden tractors that were painted to appear identical to Perkins’ metal tractors. But because they were made of wood, they could not conduct electricity.
In a series of ten patients (five treated with real, and five with fake tractors), the “placebo” tractors worked as well as the real ones. Haygarth concluded that tractors didn’t work. Interestingly, the trial did not show that the tractors did not benefit people, but merely that they did not produce their benefit via electricity. Haygarth himself admitted that the fake tractors worked very well. He attributed this to faith.
Other early examples of placebo controls tested the effects of homeopathy tablets compared with bread pills. One of these early trials revealed that doing nothing was better than both homeopathy and allopathic (standard) medicine.
By the middle of the 20th century, placebo-controlled trials were prevalent enough for Henry Knowles Beecher to produce one of the earliest examples of a “systematic review” that estimated how powerful placebo were. Beecher served in the United States Army during the second world war. Working on the front line in southern Italy, supplies of morphine were running out, and Beecher reportedly saw something that surprised him. A nurse injected a wounded soldier with saltwater instead of morphine before an operation. The soldier thought it was real morphine and didn’t appear to feel any pain.
After the war, Beecher reviewed 15 placebo-controlled trials of treatments for pain and a number of other ailments. The studies had 1,082 participants and found that, overall, 35% of the patients’ symptoms were relieved by placebo alone. In 1955, he published his study in his famous article The Powerful Placebo.
In the 1990s, researchers questioned Beecher’s estimates, based on the fact that the people who got better after taking the placebos might have recovered even if they had not taken the placebo. In philosophy-speak the possibly mistaken inference that the placebo caused the cure is called the post hoc ergo propter hoc (after, therefore because of) fallacy.
To test whether placebos really make people better, we have to compare people who take placebos with people who take no treatment at all. Danish medical researchers Asbjørn Hróbjartsson and Peter Gøtzsche did just that. They looked at three-armed trials that included active treatment, placebo control, and untreated groups. Then they checked to see whether the placebo was better than doing nothing. They found a tiny placebo effect that they said could have been an artefact of bias. They concluded that “there is little evidence that placebos, in general, have powerful clinical effects”, and published their results in an article called Is the placebo powerless?, which contrasted directly with the title of Beecher’s paper.
However, Hróbjartsson and Gøtzsche corrected Beecher’s mistake only to introduce one of their own. They included anything labelled as a placebo in a trial for any condition. Such a comparison of apples and oranges is not legitimate. If we looked at the effect of any treatment for any condition and found a tiny average effect, we could not conclude that treatments were not effective. I exposed this error in a systematic review, and now it is widely accepted that just as some treatments are effective for some things but not everything, some placebos are effective for some things – especially pain.
Placebo surgery
Recently, placebo-controlled surgery trials have been used. In perhaps the most famous of these, American surgeon Bruce Moseley found 180 patients who had such severe knee pain that even the best drugs had failed to work. He gave half of them real arthroscopy and the other half placebo arthroscopy.
Patients in the placebo arthroscopy group were given anaesthetics and a small incision was made in their knees, but there was no arthroscope, no repairing of damaged cartilage, and no cleaning out of loose fragments of bone.
To keep the patients ignorant about which group they were in, the doctors and nurses talked through a real procedure even if they were performing the placebo procedure.
The fake surgery worked as well as the “real” surgery. A review of over 50 placebo-controlled surgery trials found that placebo surgery was as good as the real surgery in more than half the trials.
Honest placebos
A placebo can work even if a patient does not believe it is a “real” treatment.
In the first of the studies of open-label placebos (placebos that patients know are placebos) I know of, two Baltimore doctors by the names of Lee Park and Uno Covi gave open-label placebos to 15 neurotic patients. They presented the placebo pills to the patients and said: “Many people with your kind of condition have been helped by what are sometimes called sugar pills and we feel that a so-called sugar pill may help you, too.”
The patients took the placebos, and many of them got better after having the placebo – even though they knew it was a placebo. However, the patients were neurotic and a bit paranoid so they didn’t believe the doctors. After the placebo made them better, they thought the doctors had lied and actually given them the real drug.
More recently, several higher-quality studies confirm that open-label placebos can work. These “honest” placebos may work because patients have a conditioned response to an encounter with their doctor. Just like an arachnophobe’s body can react negatively to a spider even if they know it’s not poisonous, someone can react positively to treatment from a doctor even if they know the doctor is giving them a sugar pill.
The history of learning how placebos work
An early study investigating the inner pharmacology of placebo mechanisms is Jon Levine and Newton Gordon’s 1978 study of 51 patients who had impacted molars extracted. All 51 patients had received a painkiller called mepivacaine for the surgical procedure. Then, at three and four hours after the surgery, the patients were given either morphine, a placebo or naloxone. The patients didn’t know which one they had received.
Naloxone is an opioid antagonist, which means that it stops drugs such as morphine and endorphins from producing their effects. It literally blocks the cell receptors, so it stops morphine (or endorphins) from docking onto those receptors. It’s used to treat morphine overdose.
The researchers found that naloxone blocked the painkilling effect of placebos. This shows that placebos cause the release of painkilling endorphins. Since then, many experiments have confirmed these results. Hundreds of others have shown that placebo treatments affect the brain and body in several ways.
The main mechanisms by which placebos are believed to work are expectancy and conditioning.
In a comprehensive study published in 1999 of conditioning and expectancy mechanisms, Martina Amanzio and Fabrizio Benedetti divided 229 participants into 12 groups. The groups were given a variety of drugs, were conditioned in a number of ways and were given different messages (to induce high or low expectancy). The study found that placebo effects were caused by both expectancy and conditioning.
Despite the progress, some researchers argue – and I agree – that there is something mysterious about how placebos work. In a personal communication, Dan Moerman, a medical anthropologist and ethnobotanist, explained it better than I can:
We know from all the MRI people that it’s easy enough to see what happens inside to the amygdala, or whatever other bit might be involved, but what moved the amygdala, well, that takes some work.
History of placebo ethics
The accepted view in clinical practice is that placebos are not ethical because they require deception. This view has not yet fully accounted for the evidence that we don’t need deception for placebos to work.
The history of the ethics of placebo controls is more complex. Now that we have many effective treatments, we can compare new treatments with proven therapies. Why would a patient agree to enrol in a trial comparing a new treatment with a placebo when they could enrol in a trial of a new treatment compared with a proven one?
Doctors who take part in such trials may be violating their ethical duty to help and avoid harm. The World Medical Association initially banned placebo-controlled trials where a proven therapy was available. Yet in 2010, they reversed this position and said we sometimes needed placebo-controlled trials, even if there is a proven therapy. They claimed there were “scientific” reasons for doing this.
These so-called scientific reasons have been presented using obscure (to most people) concepts such as “assay sensitivity” and “absolute effect size”. In plain English, they boil down to two (mistaken) claims:
They say we can only trust placebo controls. This was true in the past. Historically, treatments like bloodletting and cocaine were used to treat a number of ailments yet were often harmful. Say we’d done a trial comparing bloodletting with cocaine for anxiety, and it turned out bloodletting was better than cocaine. We couldn’t infer that bloodletting was effective: it could have been worse than a placebo or doing nothing. In these historical cases, it would have been better to compare those treatments against a placebo. But now, we have effective treatments that can be used as benchmarks. So if a new drug came along for treating anxiety, we could compare it with the proven effective treatment. If the new treatment proved to be at least as good as the old one, we could say it is effective.
They say only placebo controls provide a constant baseline. This is based on the mistaken view that placebo treatments are “inert” and therefore have constant, invariable effects. This, too, is mistaken. In a systematic review of placebo pills in ulcer trials, the placebo response ranged from 0% (not having any effect) to 100% (complete cure).
As the arguments supporting placebo-controlled trials are being questioned, there is now a movement urging the World Medical Association needs to do another U-turn, back to its original position.
Whither placebo?
For centuries, the word “placebo” was closely linked to deception and pleasing people. Recent studies of open-label placebos show that they need not be deceptive to work. Contrariwise, studies of placebos show that they are not inert or invariable and the basis for the current World Medical Association position has been undermined. The recent history of placebos seems to pave the way for more placebo treatments in clinical practice and fewer in clinical trials.
I acknowledge the James Lind Library, the writing of Ted Kaptchuk, Jeffrey Aronson, and the mentorship of Dan Moerman.

